Stable ambroxol hydrochloride injection and preparation method thereof
A technology for the preparation of ambroxol hydrochloride and injections, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve problems such as differences in the content of insoluble particles, and reduce production costs. The effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
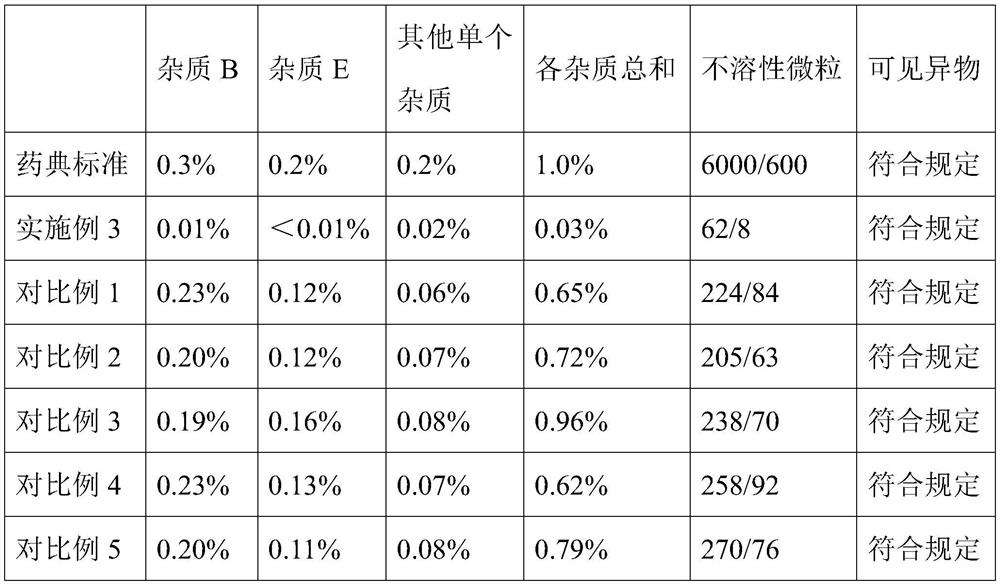
[0053] A stable ambroxol hydrochloride injection, comprising: ambroxol hydrochloride 7.5g / L, disodium hydrogen phosphate 1.8g / L, citric acid 1.5g / L, methionine 0.3g / L, edetate dicalcium 0.05g / L.
[0054] The above-mentioned stable preparation method of ambroxol hydrochloride injection comprises the following steps: dissolving disodium hydrogen phosphate, citric acid, methionine and dicalcium edetate in water to make the pH value of the system 6.0, then adding ambroxol hydrochloride The cable was completely dissolved, then glucose was added to adjust the osmotic pressure to isotonicity, constant volume, and then filtered with a 0.22 μm filter membrane, filled with nitrogen, and sterilized at 120°C for 20 minutes.
Embodiment 2
[0056] A stable ambroxol hydrochloride injection, comprising: ambroxol hydrochloride 7.5g / L, disodium hydrogen phosphate 2.2g / L, citric acid 0.66g / L, methionine 0.1g / L, edetate dicalcium 0.1g / L.
[0057] The above-mentioned stable preparation method of ambroxol hydrochloride injection comprises the following steps: dissolving disodium hydrogen phosphate, citric acid, methionine and dicalcium edetate in water to make the system pH value 4.0, then adding ambroxol hydrochloride The cable was completely dissolved, then glucose was added to adjust the osmotic pressure to isotonicity, constant volume, and then filtered with a 0.22 μm filter membrane, filled with nitrogen, and sterilized at 125°C for 15 minutes.
Embodiment 3
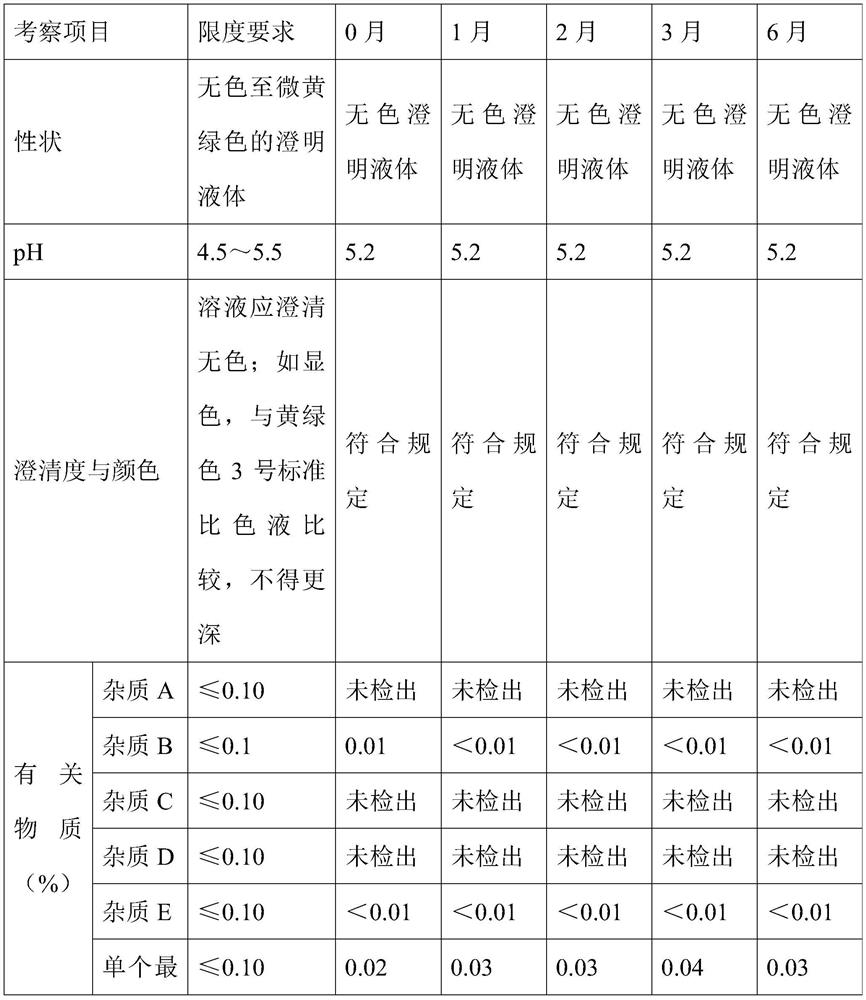
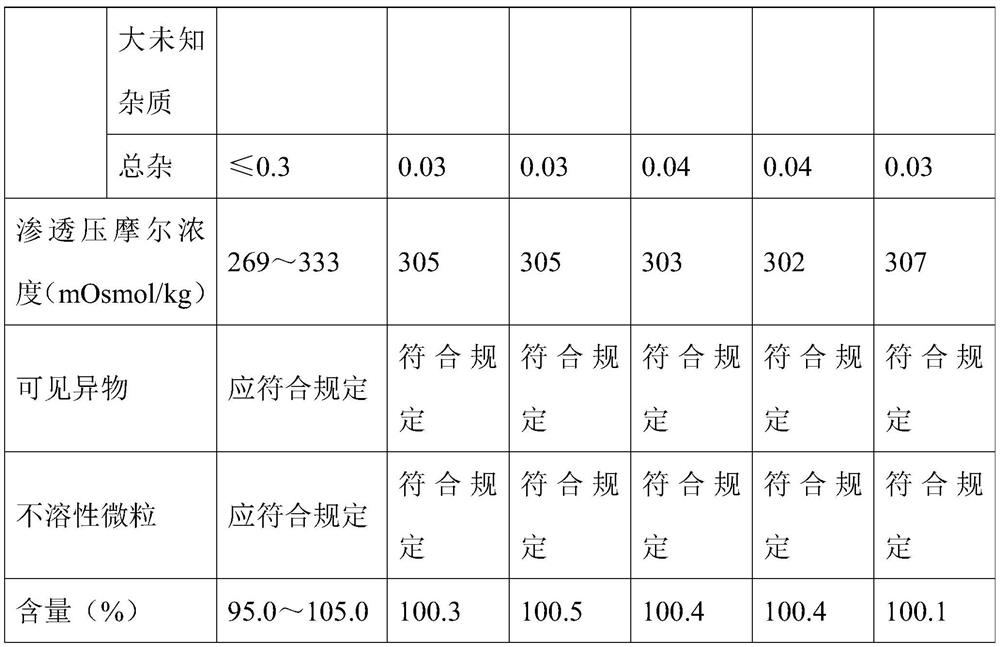
[0059] A stable ambroxol hydrochloride injection, comprising: ambroxol hydrochloride 7.5g / L, disodium hydrogen phosphate 2.0g / L, citric acid 0.75g / L, cysteine 0.3g / L, edetine Disodium acid 0.1g / L, sodium chloride 7g / L.
[0060] The preparation method of above-mentioned stable ambroxol hydrochloride injection comprises the steps of: adding disodium hydrogen phosphate, citric acid, cysteine and edetate disodium into water and dissolving completely, making the system pH value 5.0~5.5, Then add ambroxol hydrochloride to completely dissolve, then add sodium chloride to adjust the osmotic pressure, dilute to 1000mL with water for injection, then filter twice with a 0.22μm filter membrane, fill it with nitrogen and seal it in a 2mL brown ampoule, 121 Sterilize at ℃ for 15 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap