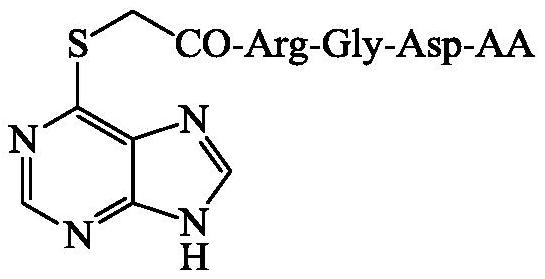
6-(acetyl-Arg-Gly-Asp-AA-sulfydryl)purine, and synthesis, activity and application thereof
A technology of -gly-asp, mercaptopurine, applied in the preparation of anti-tumor drugs, anti-tumor activity, 6-purine field, can solve problems such as no obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
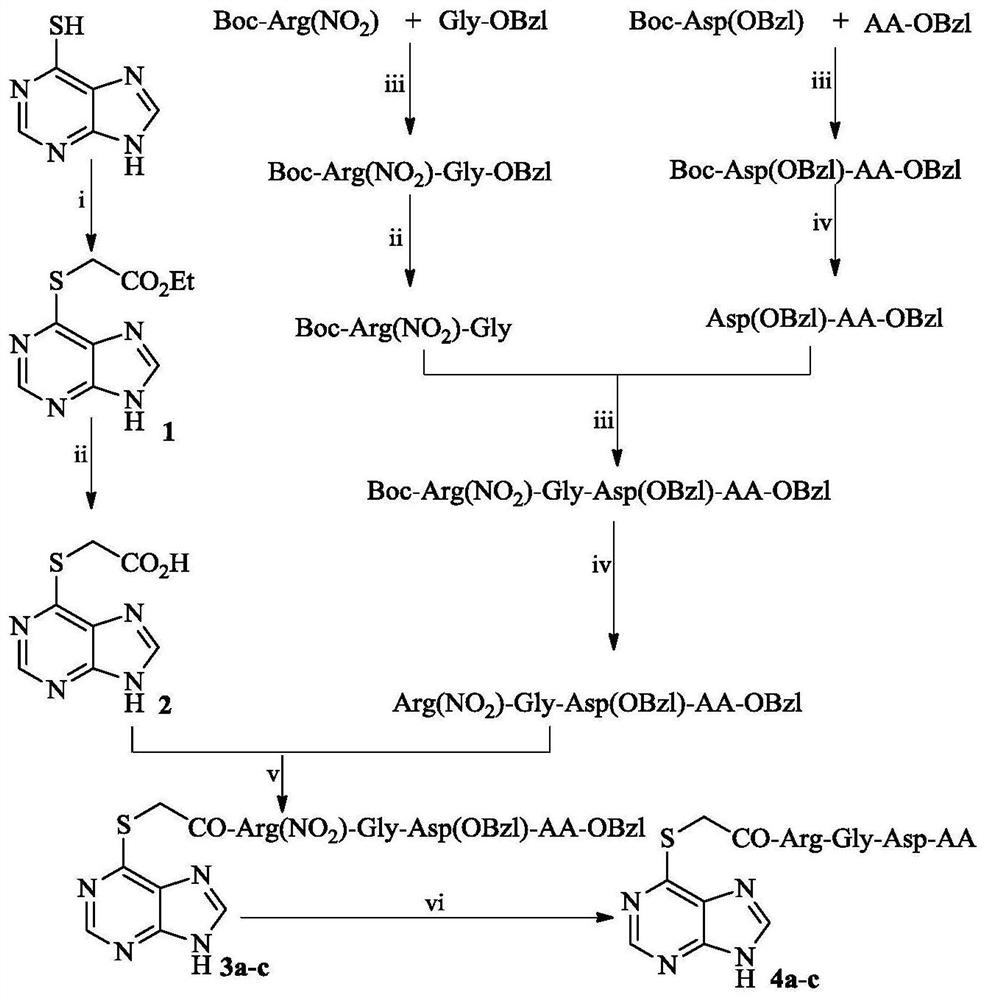
[0019] Embodiment 1 prepares 6-(acetyl-O-ethylmercapto) purine (1)
[0020] Add 50 mL of N,N-dimethylformamide to 2.070 g (13.60 mmol) of 6-mercaptopurine, and stir at 65°C until 6-mercaptopurine is completely dissolved, and the solution is yellow and clear. Add 2.250g (16.32mmol) K 2 CO 3 As a catalyst, stir for 30min, add 1.80mL (16.32mmol) ethyl bromoacetate, and continue the reaction at 65°C. After 24 hours, TLC (petroleum ether / ethyl acetate=1 / 2) showed that 6-mercaptopurine disappeared, the reaction solution was filtered, and the filtrate was concentrated under reduced pressure. The obtained orange oil was purified by silica gel column chromatography (petroleum ether / ethyl acetate=1 / 1) to obtain 2.360 g (72%) of the title compound as a colorless solid. ESI-MS(m / e):239[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 ):δ / ppm=13.651(s,1H),8.503(s,1H),8.499(s,1H),4.175(q,J=7.8Hz,2H),4.036(dd,J 1 =12.0Hz,J 2 =5.4Hz, 1H), 1.254(t, J=7.8Hz, 3H); 13 C-NMR (75MHz, DMSO-d 6 ): δ / ppm=...
Embodiment 2
[0021] Embodiment 2 prepares 6-(carboxymethylmercapto) purine (2)
[0022] 0.310g (1.30mmol) of 6-(acetyl-O-ethylmercapto)purine (1) was completely dissolved in 10mL of methanol, and the solution was clear and transparent. The pH of the solution was adjusted to 13 with 2N NaOH aqueous solution at 0°C. After stirring at 0°C for 4 h, the reaction was complete as monitored by TLC (petroleum ether / ethyl acetate=1 / 2). The reaction solution was saturated KHSO 4 The aqueous solution was adjusted to pH 7, concentrated under reduced pressure, a colorless salt solid precipitated, and 5 mL of water was added to completely dissolve the solid. The solution was heated at 0°C with saturated KHSO 4 The pH of the aqueous solution was adjusted to 2, and the solid was fully separated out after standing, filtered, and the filter residue was washed with distilled water, and allowed to dry naturally at room temperature to obtain 0.245 g (89%) of the title compound as a colorless solid. ESI-MS(m / ...
Embodiment 3
[0023] Example 3 Preparation of Boc-Asp(OBzl)-Phe-OBzl
[0024] Dissolve 1.660g (5.00mmol) Boc-Asp (OBzl) in 30mL of anhydrous tetrahydrofuran, add 0.675g (5.00mmol) 1-hydroxybenzotriazole at 0°C, stir for 10min, then add 1.130g (5.50mmol) dihydrofuran Cyclohexylcarbodiimide, stirred for 30min. Add 2.360g (5.50mmol) of Phe-OBzl to the reaction solution at 0°C, adjust the pH of the reaction mixture to 9 with N-methylmorpholine, and stir at room temperature for 12h. TLC (dichloromethane / methanol=40 / 1) shows The response is complete. The reaction solution was filtered, the filtrate was concentrated under reduced pressure, the residue was dissolved in 100mL ethyl acetate, the insoluble matter was filtered off, and the filtrate was washed with saturated NaHCO 3 Wash with aqueous solution (30mL×3), wash with saturated NaCl aqueous solution (30mL×3), 5% KHSO 4 Wash with aqueous solution (30mL×3), wash with saturated NaCl aqueous solution (30mL×3), and wash with saturated NaHCO 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com