CD4 positive cell specific gene transfer vector and application thereof
A positive cell and specific technology, applied in the field of molecular pharmacology and molecular medicine, can solve the problems of unsatisfactory modification efficiency of specific targeting CD4 positive cells, reduction of rAAV vector production efficiency, and difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Plasmid pAAV is a plasmid carrying the whole gene of wild-type AAV2 virus (pCap / Rep, a gift from WeidongXiao Laboratory, Temple University, USA), wherein the Cap gene is responsible for encoding the capsid protein of AAV2 virus.
[0031] (1) Primer design
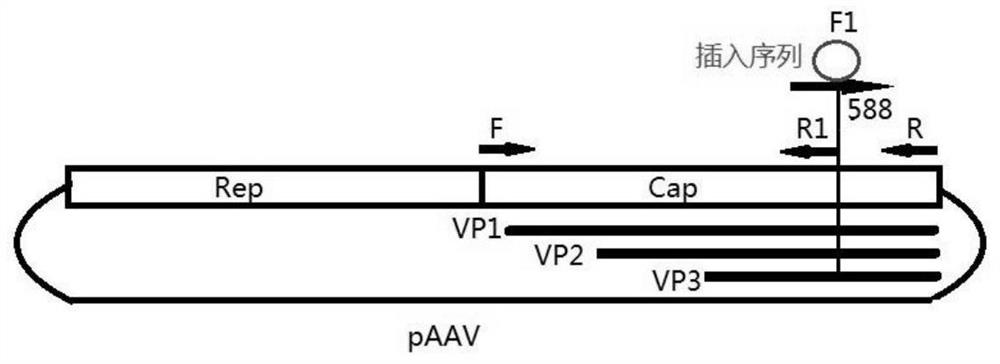
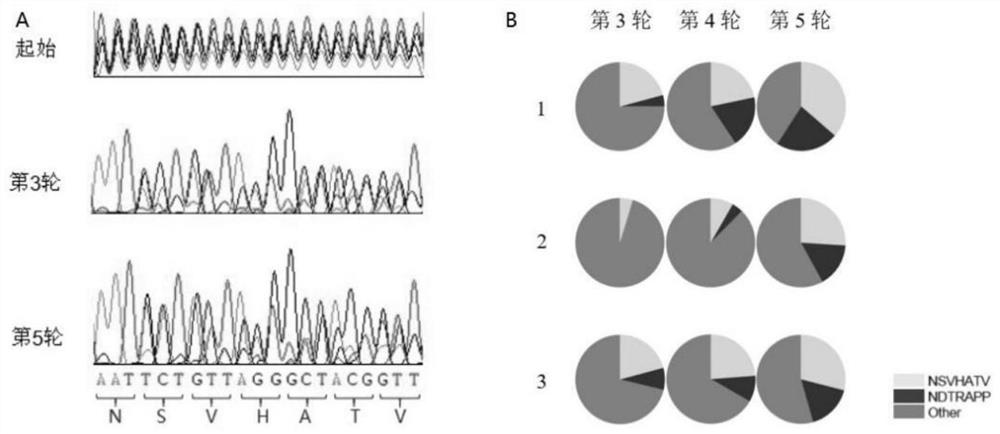
[0032] Such as figure 1 As shown, a total of 4 PCR primers for modifying AAV capsid were designed, as shown in SED ID NO: 27-30:
[0033] Primer F (TCAGGTGTTTACTGACTCGGAG, SED ID NO: 27);
[0034] Primer R (CGTCACACATACGACACCGG, SED ID NO: 28);
[0035] Primer R1 (TCTGTTGCCTCTCTGGAGGTTG, SED ID NO: 29);
[0036] Primer F1 (CAACCTCCAGAGAGGCAACAGANNNNNNNNNNNNNNNNNNNCAAGCAGCTACCGCAGATGTC, SED ID NO: 30), wherein 21 Ns refer to the inserted random fragment of 21 bases.
[0037] (2) Preparation of Fragment 1
[0038] Fragment 1 was amplified by PCR using plasmid pAAV as a template and primers F and R1 as paired primers.
[0039] (3) Preparation of Fragment 2
[0040] Fragment 2 was amplified by PCR using plasmid pA...
Embodiment 2
[0051] (1) Preparation of AAV capsid mutant library
[0052] 293 cells were inoculated in culture dishes and cultured overnight in DMEM medium containing 10% FBS until the confluence was about 80%.
[0053] Take 1 mL serum-free medium, add 8 μg pAAV-lib and 10 μg helper plasmid pFd6, mix gently, then add 1 mg / mL PEI solution at a ratio of 1:3, and vortex to mix. Add dropwise evenly to the culture medium of 293 cells, and at the same time shake gently to mix as soon as possible.
[0054] Place cells at 37 °C, 5% CO 2 16 hours after transfection, the old medium was discarded, and 10 mL of fresh DMEM medium containing 10% FBS was added.
[0055] Continue culturing in the cell incubator for 72 hours, collect the cells into a 15mL centrifuge tube, centrifuge at 1000g, 4°C for 15min, store the supernatant at 4°C, and resuspend the pellet with 1mL PBS solution into a 1.5mL centrifuge tube.
[0056] The collected cell suspension was frozen in a -80°C refrigerator for 1 hour, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com