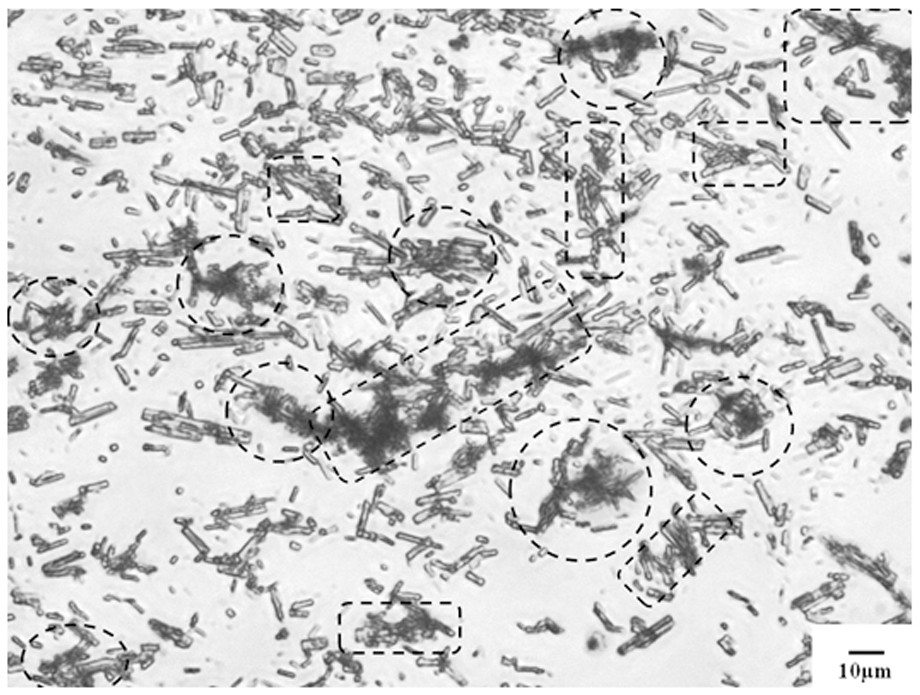
Pharmaceutical composition containing pyrroloquinoline quinone trilithium salt nonahydrate, capsule and preparation method thereof
A technology of pyrroloquinoline quinone and hydrate, which is applied in the field of pharmaceutical preparations, can solve the problems of reduced production efficiency, impact on the stability of the crystal form of pyrroloquinoline quinone trilithium salt nonahydrate, and difficulties in subsequent processing, so as to improve production efficiency and yield, good stability and dissolution effect, and solve the effect of easy aggregation and agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to Embodiment 4
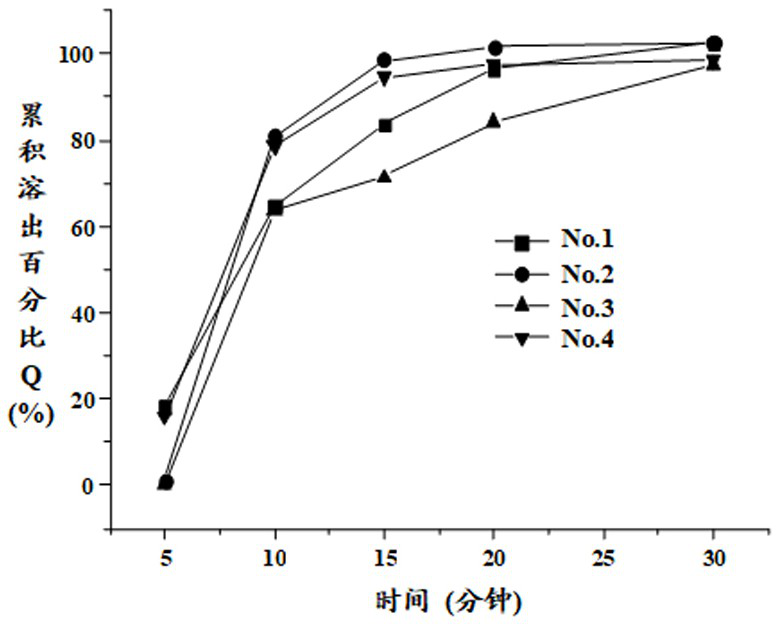
[0065] Examples 1 to 4 Compositions, Capsules and Preparations Containing Pyrroloquinoline Quinone Trilithium Salt Nonahydrate
[0066] The prescription composition of table 1 embodiment 1
[0067]
[0068] Note: The contents prepared by prescription No.1, No.2, No.3 and No.4 are poured into No. 3, No. 3, No. 4 and No. 3 hypromellose hollow capsules respectively.
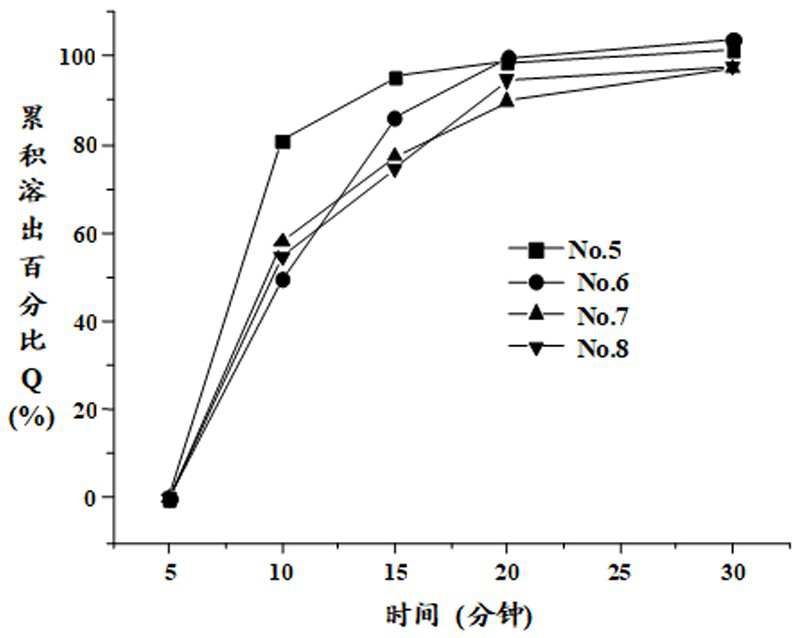
[0069] The prescription composition of table 2 embodiment 2
[0070]
[0071] Note: The contents prepared by prescription No.5, No.6, No.7 and No.8 are poured into No. 4, No. 3, No. 3 and No. 3 hypromellose hollow capsules respectively.
[0072] The prescription composition of table 3 embodiment 3
[0073]
[0074] Note: The contents prepared by prescription No.9, No.10 and No.12 are poured into No.4, No.4 and No.2 hypromellose hollow capsules respectively. The contents prepared by prescription No.11 are poured into No. 2 gelatin hollow capsules.
[0075] The prescription composition of table 4 embodime...
Embodiment 1
[0079] Example 1 (Prescription No.1~No.4), Example 2 (Prescription No.5~No.8), Example 3 (Prescription No.9~No.12), Example 4 (Prescription No.13 and prescription No.15) preparation method:
[0080] (1) Raw material pretreatment: sieve the raw material of pyrroloquinoline quinone trilithium salt nonahydrate with 30 meshes, and sieve the excipients with 40 meshes;
[0081] (2) Ingredients: According to the proportion of the process prescription, weigh the raw material drug and auxiliary materials of pyrroloquinoline quinone trilithium salt nonahydrate;
[0082] (3) Pre-mixing: add pyrroloquinoline quinone trilithium salt nonahydrate API and all excipients into a multi-directional motion mixer, and mix at a speed of 20 rpm for 15 minutes to make the mixture uniform;
[0083] (4) Dry granulation: Add the homogeneously mixed pyrroloquinoline quinone trilithium salt nonahydrate API and all excipients to the dry granulator at a temperature of 15°C to 25°C and a relative humidity of...
Embodiment 4
[0088] The preparation method of embodiment 4 (prescription No.14 and prescription No.16):
[0089] (1) Raw material pretreatment: sieve the raw material of pyrroloquinoline quinone trilithium salt nonahydrate with 30 meshes, and sieve the excipients with 40 meshes;
[0090] (2) Ingredients: According to the proportion of the process prescription, weigh the raw material drug and excipients of pyrroloquinoline quinone trilithium salt nonahydrate, including internally added excipients and externally added excipients;
[0091] (3) Pre-mixing: add pyrroloquinoline quinone trilithium salt nonahydrate API and internally added excipients into a multi-directional motion mixer, and mix at a speed of 20 rpm for 15 minutes to make the mixture uniform;
[0092] (4) Dry granulation: at a temperature of 15°C to 25°C and a relative humidity of 45% to 55%, add the evenly mixed raw material drug of pyrroloquinoline quinone trilithium salt nonahydrate and internally added excipients to the dry ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap