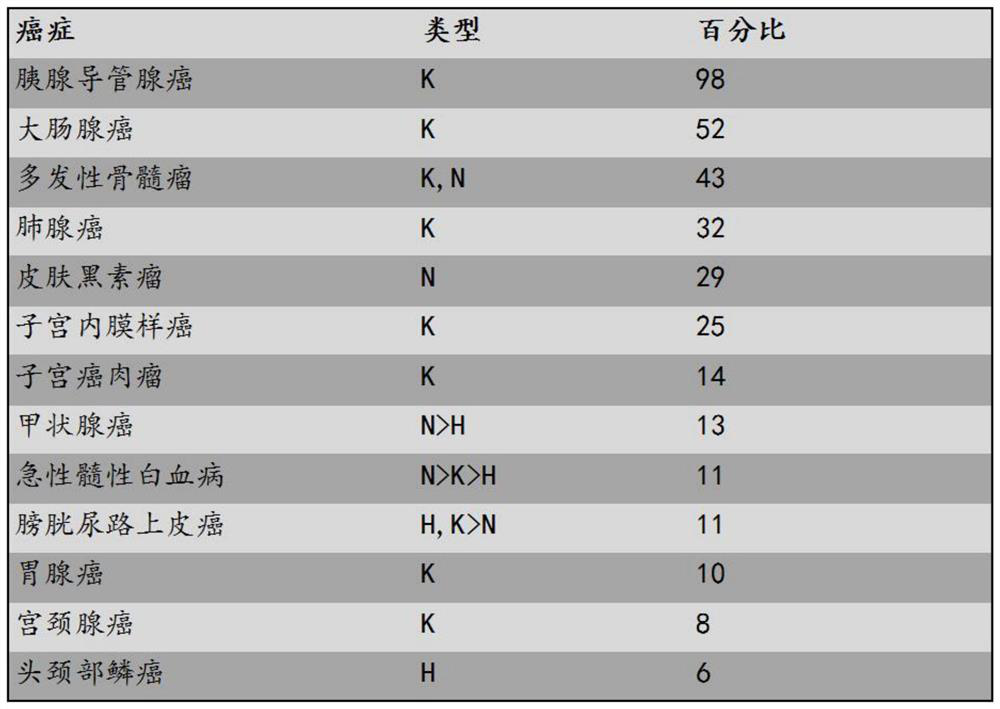
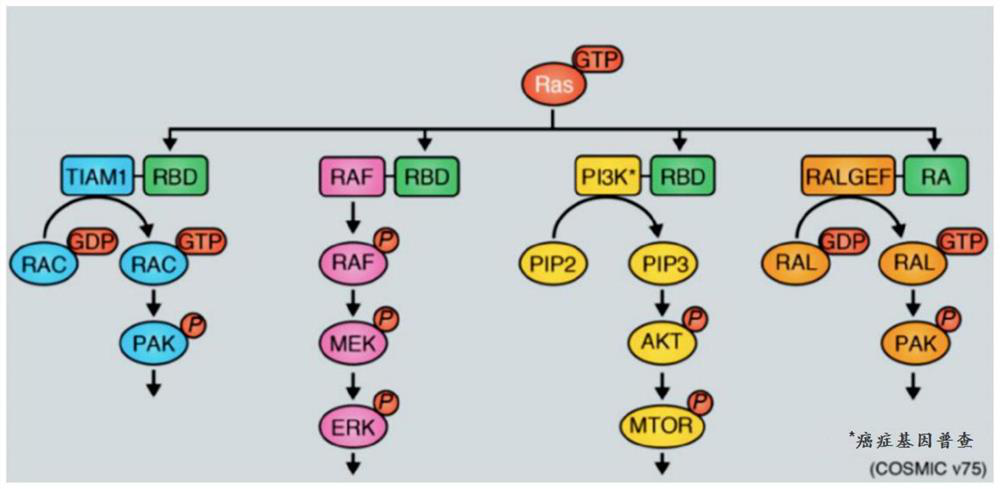
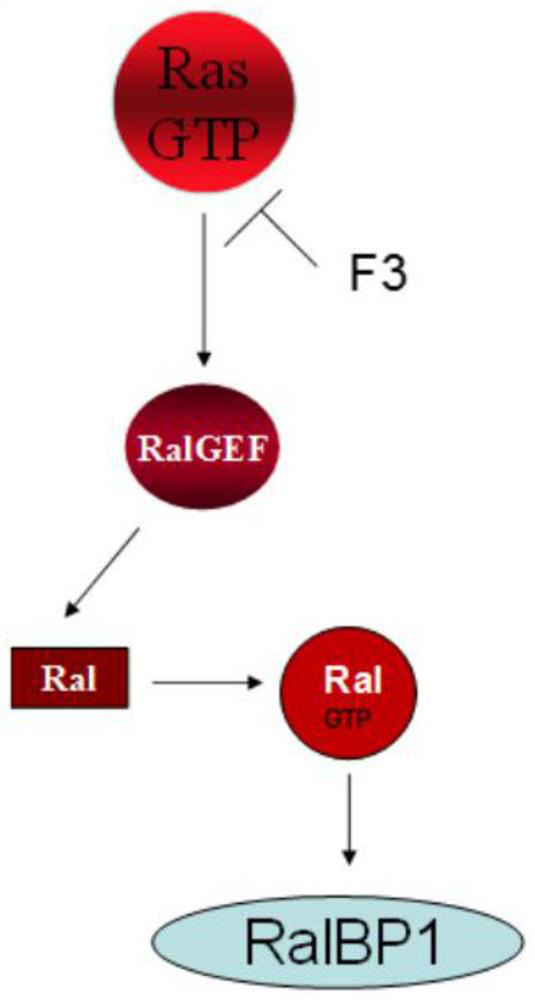
Inhibitors of ras oncoprotein, methods of making and methods of use thereof
An alkyl, disease-based technology, applied in the field of RAS oncoprotein inhibitors and its preparation and use, can solve problems such as unapproved treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1: the synthesis of compound
[0093] The corresponding amine R can be prepared by first 1 -NH 2 and R 2 -NH 2 , then with the diacid X 1 (CH 2 COOH) 2 Sequential reactions to prepare compounds of formula I, diacid X 1 (CH 2 COOH) 2 May optionally be in the form of a cyclic anhydride. In the examples below, R 1 and R 2 Contains substituted phenyl groups. In the first example, the substituted phenyl groups are labeled "A" and "B", respectively.
[0094]
[0095] Synthesis of sulfonamide fragments;
[0096]
[0097] Scheme 1. Synthesis of F3 sulfonamide fragment.
[0098] The synthesis of F3 starts with the construction of sulfonamide 4 (B ring of F3). The sulfonamide was prepared by treating commercially available sulfonyl chloride 1 with two equivalents of amine 2. Reduction of the nitro group 3 under catalytic hydrogenation conditions completed the synthesis in excellent overall yields. Various sulfonamide derivatives are prepared by s...
Embodiment 2
[0138] Embodiment 2: the test of compound
[0139] Binding test
[0140] Direct binding of F3 / F3a derivatives to recombinant K-RAS protein. Thermal fluorescence analysis was used to determine whether there was a direct interaction between the recombinant K-RAS protein and compound F3, or between the recombinant K-RAS protein and compound JAB-6-5. Recombinant K-RAS12v protein was loaded with γGTPS and used for binding assays at a ratio of 200 μM compound / 5 μM protein. Control compound C4 targets a different protein and is not expected to bind RAS. The assay results of the three derivatives were performed in triplicate. With the exception of C4, significant curve shifts were observed for both F3 and JAB-6-5. From the curve it can be deduced that the predicted kD is about 25 μM at 55.5°C. The result is as Figure 16 shown.
[0141] Three-dimensional soft agar drug screening (in vitro test)
[0142] The vast majority of solid tumors arise from epithelial cells. Normal epi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com