Application of traditional Chinese medicine composition in preparation of drugs for adjuvant therapy of novel coronavirus pneumonia
A coronavirus and adjuvant treatment technology, applied in drug combinations, antiviral agents, pharmaceutical formulations, etc., can solve the difficulties in the prevention and treatment of new coronavirus pneumonia, and the lack of breakthroughs in the development of antiviral drugs. Promote the absorption of lung inflammation, quickly stabilize the condition, and use safe and reliable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The following clearly and completely describes the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention. The materials, reagents, etc. used are commercially available reagents and materials unless otherwise specified.
[0026] In the traditional Chinese medicine prescription mentioned in the description of the present invention, each medicinal material is all with g as the weight measurement unit. However, those skilled in the art can understand that the same effect can be obtained by proportioning each medicinal material in the prescription in the same weight ratio.
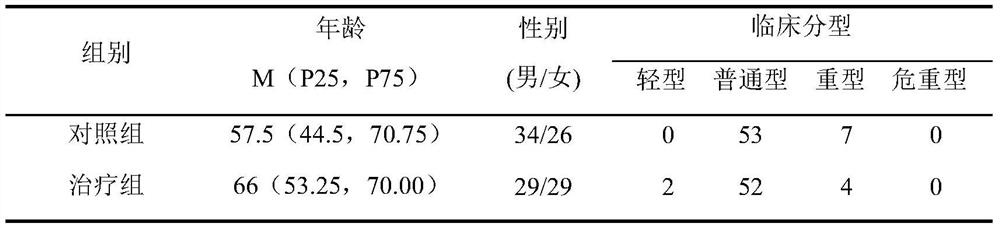
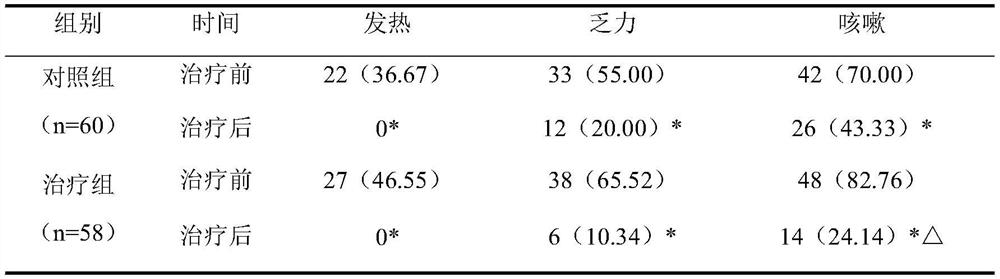
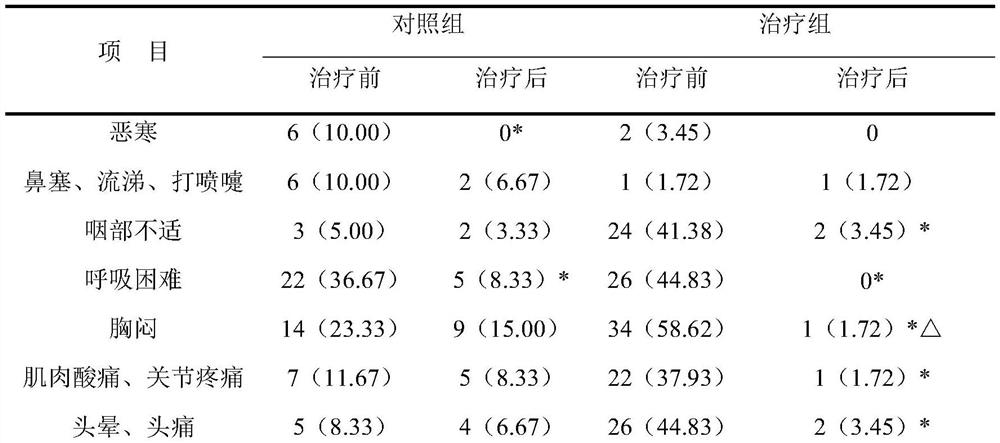
[0027] Clinical research on the adjuvant treatment of novel coronav...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com