Antibodies that target HIV gp120 and methods of use
A HIV-1 and antibody technology, applied in chemical instruments and methods, antibodies, antibody medical components, etc., can solve the problems of virus coverage, pharmacokinetics, multi-specificity and other characteristic limitations of antibody therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0415] Example 1: ADCC Activity of Antibody A
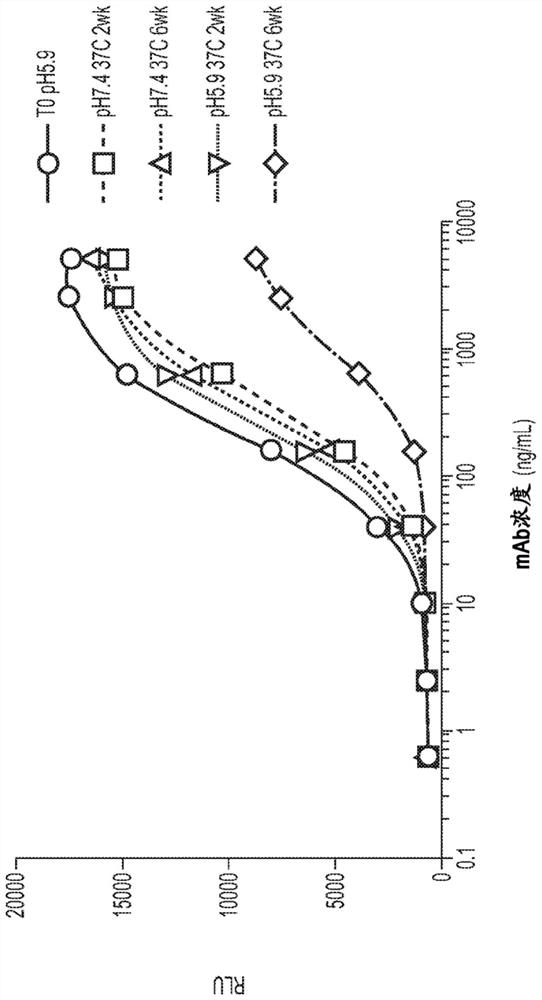
[0416] Use of HIV-infected CEM.NKr.CCR5 + Luc + cells and primary human NK effector cells from independent healthy donors were assayed in vitro for antibodies against the HIV-infected target CD4 + ADCC of T cells.
[0417] The studies included both PGT121-sensitive and PGT121-resistant viruses as well as antibodies with modifications to the Fc of Antibody A (Fc modifications). Table 1 summarizes the presence of 5 mg / mL human serum IgG and using primary human NK cells from three independent human donors and CEM.NKr.CCR5 infected with virus isolates 92US712 or 92US657 + Luc + Lethality and potency of antibodies A, A-1, A-2, A-3, A-4, A-5 and A-6 when assayed in cells.
[0418] Table 1. ADCC activity
[0419]
[0420] For a dose response of Emax 50 Annotated as >100 μg / mL.
[0421] The Fc-modified antibody showed increased killing of HIV-1-infected target CD4 T cells compared to antibody A in vitro by primary human NK c...
Embodiment 2
[0448] Example 2: Antibody Campaign (Campaign)
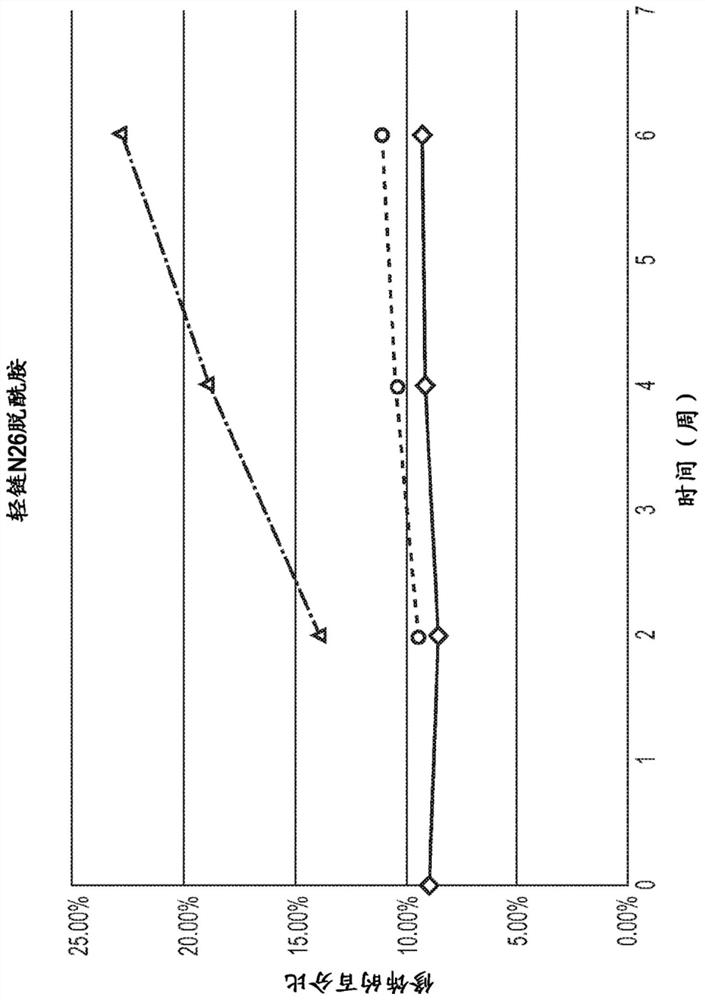
[0449] Comparison of the sequences of Antibody A and Antibody B to the human germline revealed several mutations, insertions and deletions, both internal and external to the CDRs. Briefly, a continuum of germline mismatches in the heavy chain framework region 3 (HCFR3) was identified at positions 72-78 of the heavy chain (HC). Four amino acid insertions were identified between positions 74 and 75 of HC FR3. A germline deletion was identified in CDR L1 at positions 27-30 of the light chain (LC). A continuum of germline mismatches was identified at positions 65-77 in the light chain framework region 3 (LC FR3). An N72-linked consensus glycosylation motif was identified at positions 72–74 in LC FR3. A germline deletion in CDR L3 was identified at positions 92-95. Two residues (F98 and G99) that are highly conserved in human IgG light chains were mutated in both Antibody A and Antibody B.
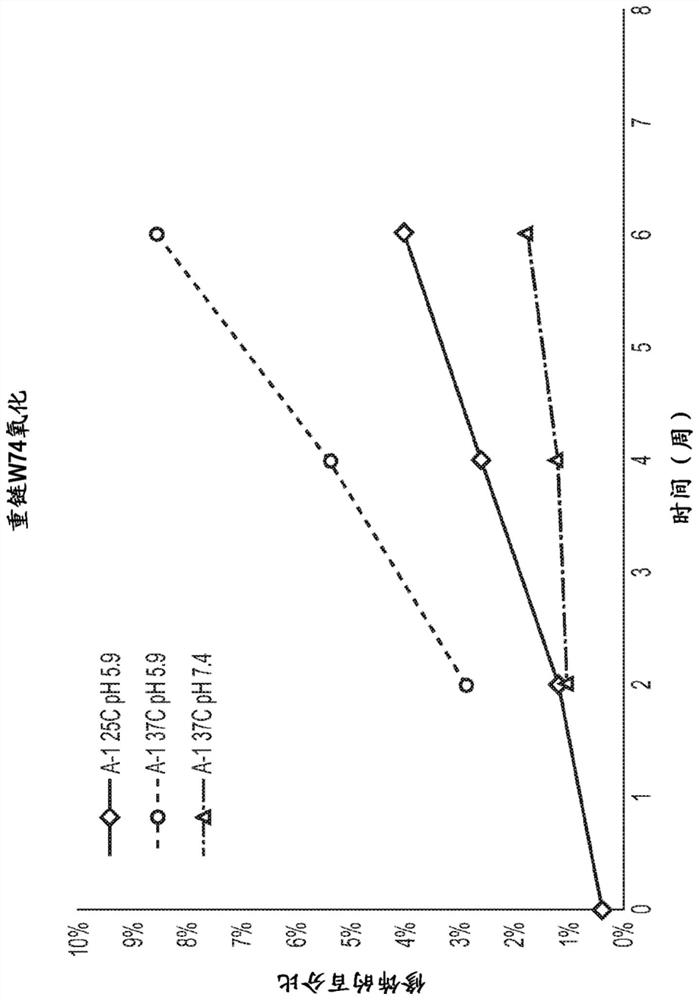
[0450]Mass spectrometry studies wer...
Embodiment 3
[0471] Embodiment 3: mass spectrometry
[0472] Antibody A-1 was transiently expressed in ExpiCHO cells and protein A purified using standard methods. Samples were denatured and reduced by using 4M guanidine hydrochloride and 50 mM DTT (final concentration) and heating at 60°C for 20 minutes. Samples were desalted on-line while the reduced heavy and light chains were separated on a BEH C4 reversed-phase column (before injection into the source of a Waters Synapt G2Si hybrid time-of-flight mass spectrometer). Packets of multiply charged protein peaks were deconvoluted using the maximum entropy deconvolution algorithm. The results show that the Antibody A light chain is glycosylated. The observed light chain mass spectra revealed the presence of G0-glycan modifications and other glycan-related mass heterogeneity. This observation is consistent with the presence of a consensus glycosylation motif at N72 in the antibody AVL domain (NLT) and a previous crystal structure of ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com