Clinical test report automatic input method and device, electronic equipment and storage medium
A technology for clinical trials and test reports, applied in the computer field, can solve problems such as low accuracy, reduce impact, improve efficiency, and reduce dependence on manual operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] Example embodiments will now be described more fully with reference to the accompanying drawings. Example embodiments may, however, be embodied in many forms and should not be construed as limited to the examples set forth herein; rather, these embodiments are provided so that this disclosure will be thorough and complete and will fully convey the concept of example embodiments to those skilled in the art. The described features, structures, or characteristics may be combined in any suitable manner in one or more embodiments.
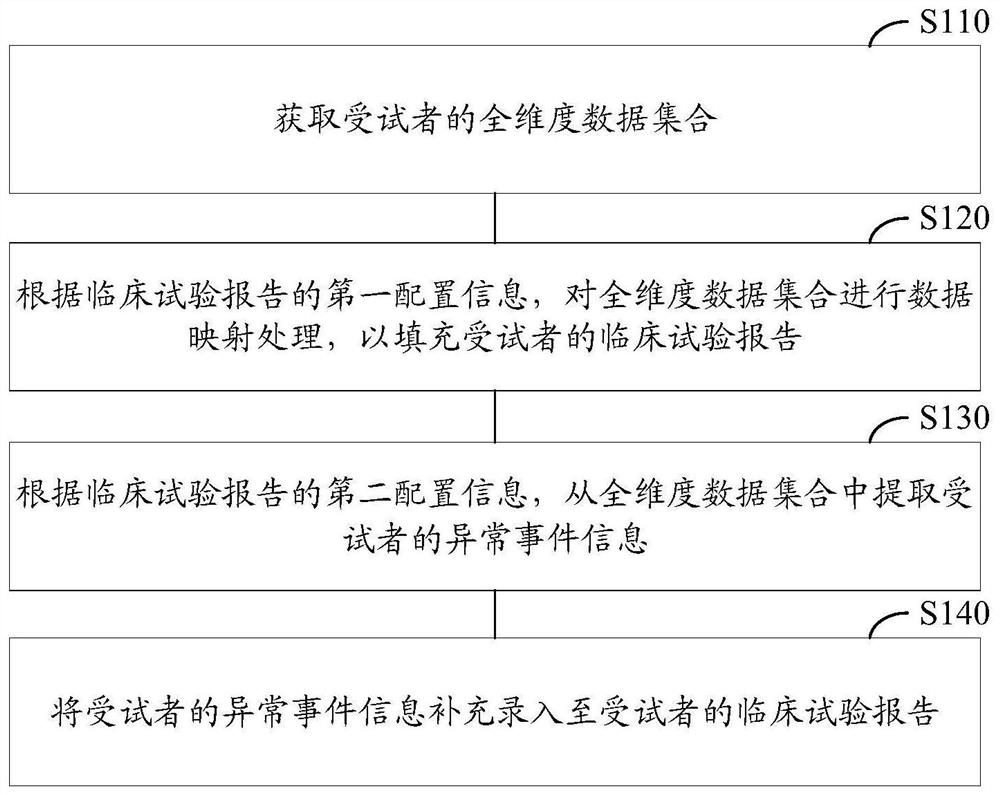
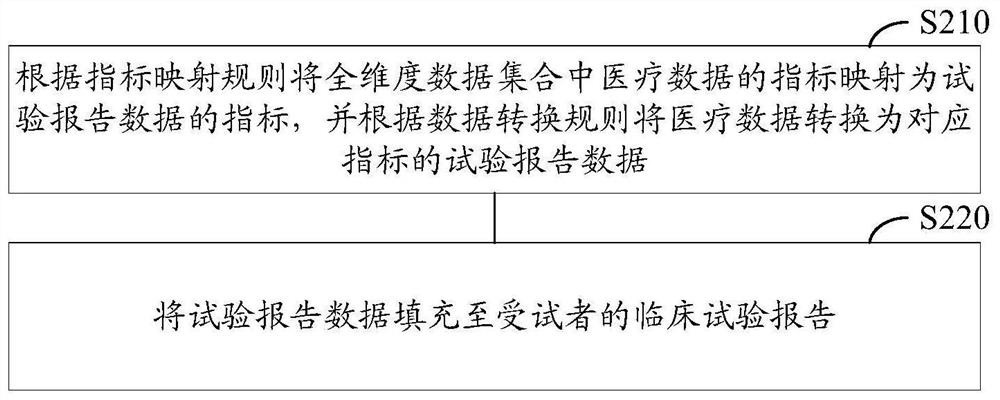
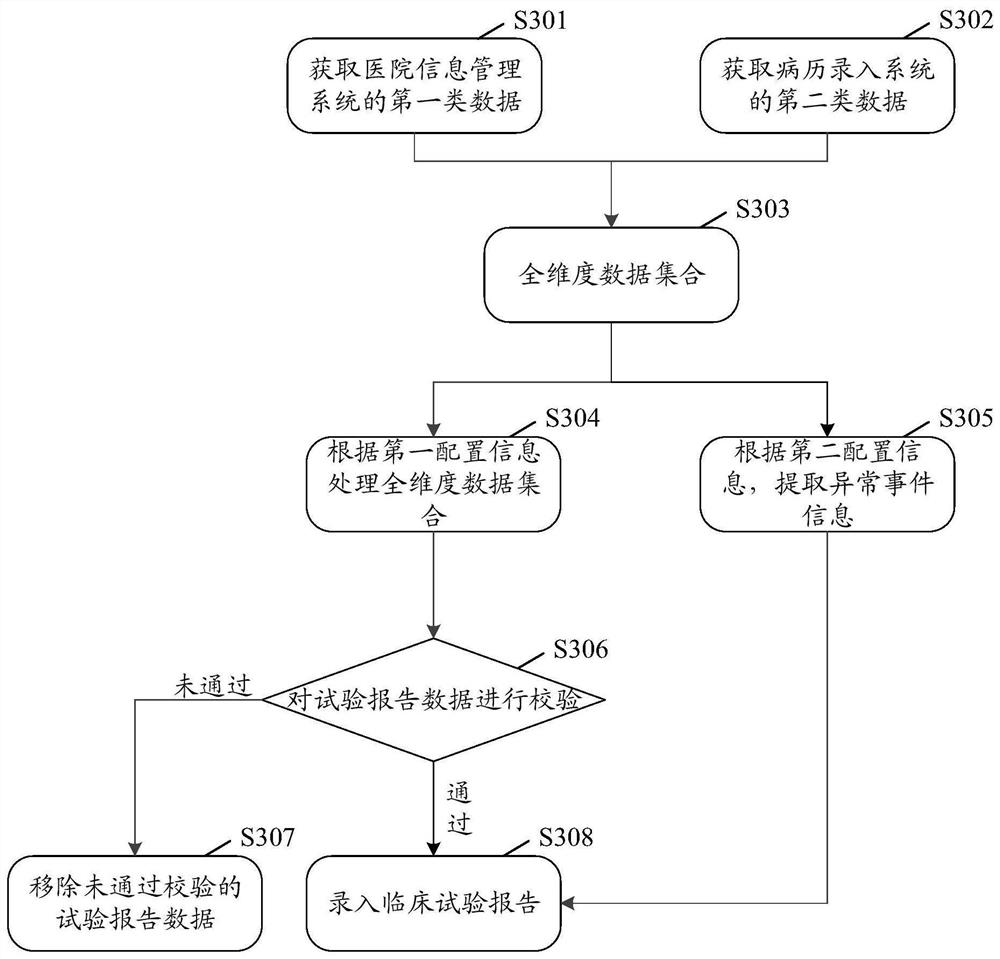
[0033]Exemplary embodiments of the present disclosure firstly provide a method for automatically entering a clinical trial report, which can be used to generate a clinical trial report about a subject, wherein the clinical trial report refers to the basic information of the subject, the treatment plan, and Record and report of treatment process and other information. The application scenario of this exemplary embodiment may be: the method of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com