Pharmaceutical composition for regulating and controlling reserve of primordial follicle
A technology of primordial follicles and compositions, applied in drug combinations, active ingredients of hydroxyl compounds, pharmaceutical formulas, etc., can solve problems such as unreported treatment methods, and achieve the effects of avoiding the risk of birth defects, maintaining fertility, and delaying decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
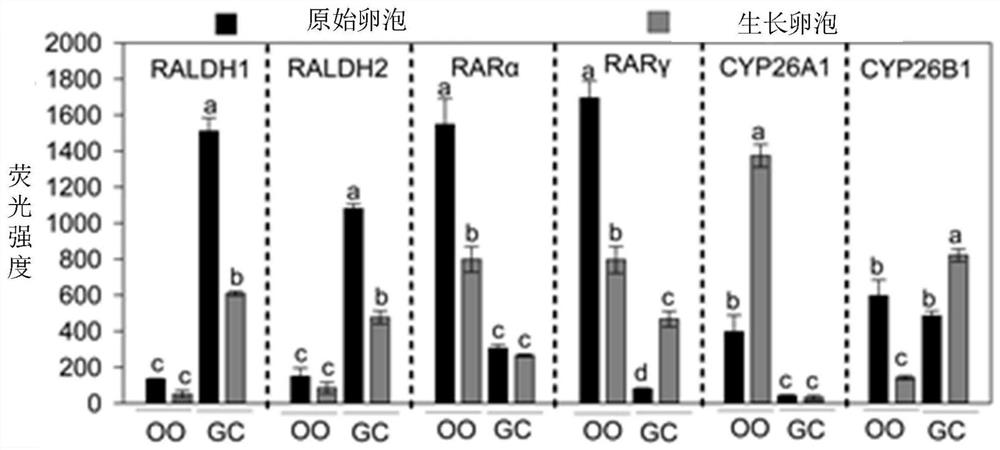
[0060] Example 1. Expression pattern of ATRA synthetase, receptor and catabolic enzyme in neonatal mouse ovary
[0061] First, the expression characteristics of ATRA signaling molecules in primordial follicles were detected by RT-qPCR, immunofluorescence and Western blot methods, and it was found that ATRA synthetase was mainly expressed in pregranulosa cells; ATRA receptors were mainly expressed in oocytes; ATRA The degrading enzymes are expressed in both pregranulosa cells and oocytes ( figure 1 ).
Embodiment 2
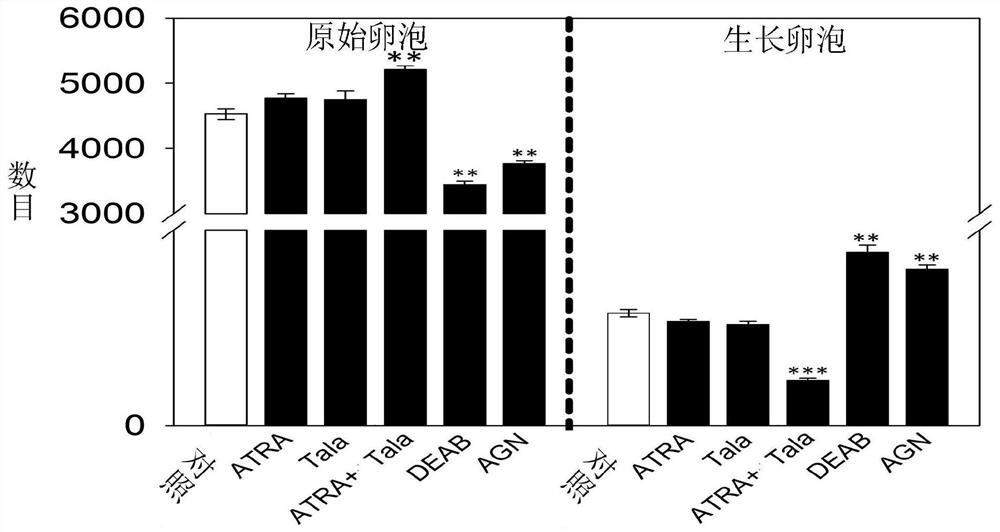
[0062] Example 2.ATRA inhibits the activation of neonatal mouse ovarian primordial follicles
[0063] In order to study the effect of ATRA on the activation of primordial follicles, the ovaries of 3dpp neonatal mice were cultured for 4 days with or without drug addition, and the ovarian structure was observed. Statistical analysis of serial sections showed that ATRA could significantly inhibit small The activation of primordial follicles in mice (116.7±4.4); while ATRA synthase inhibitors (443.3±19.2) and receptor inhibitors (400.0±10.4) can significantly increase the activation of primordial follicles ( figure 2 ).
[0064] The physiological role of ATRA was further studied. The newborn mice were injected intraperitoneally with ATRA or talarazole once a day for a total of 4 days. Different from ovaries cultured in vitro, RT-qPCR and Western blot analysis showed that injection of ATRA could not increase the gene and protein levels of metabolic enzymes in mouse ovaries. St...
Embodiment 3
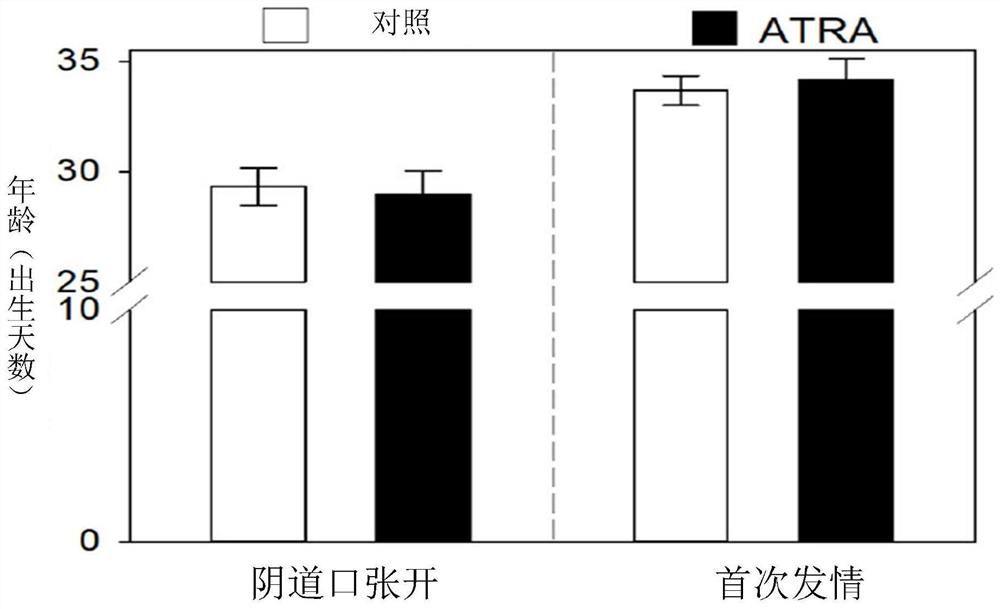
[0065] Example 3. Oral administration of ATRA can increase the number of primordial follicles in the adult mouse ovary
[0066] The effect of oral administration of ATRA on primordial follicle activation in adult mice was further tested. After serial section analysis, the number of primordial follicles (2395.0±65.6) in the 500ng / mL ATRA group was significantly higher than that in the control group (0 ng / mL group, 1996.7±54.9), and correspondingly the number of growing follicles in the 500ng / mL ATRA group (617.0 ±37.1) significantly lower than the control group (229.0±100.6) ( Figure 3a-3f ). At the same time, 500ng / mL ATRA had no significant effect on the opening of the vaginal opening, the length of the estrus cycle and the regularity of estrus in mice (P>0.05) ( Figures 3a-3c ). Therefore, 500ng / mL of ATRA is an appropriate oral concentration, and it was chosen for subsequent experiments. Next, the effect of oral administration of ATRA for 1 to 5 months on follicular d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com