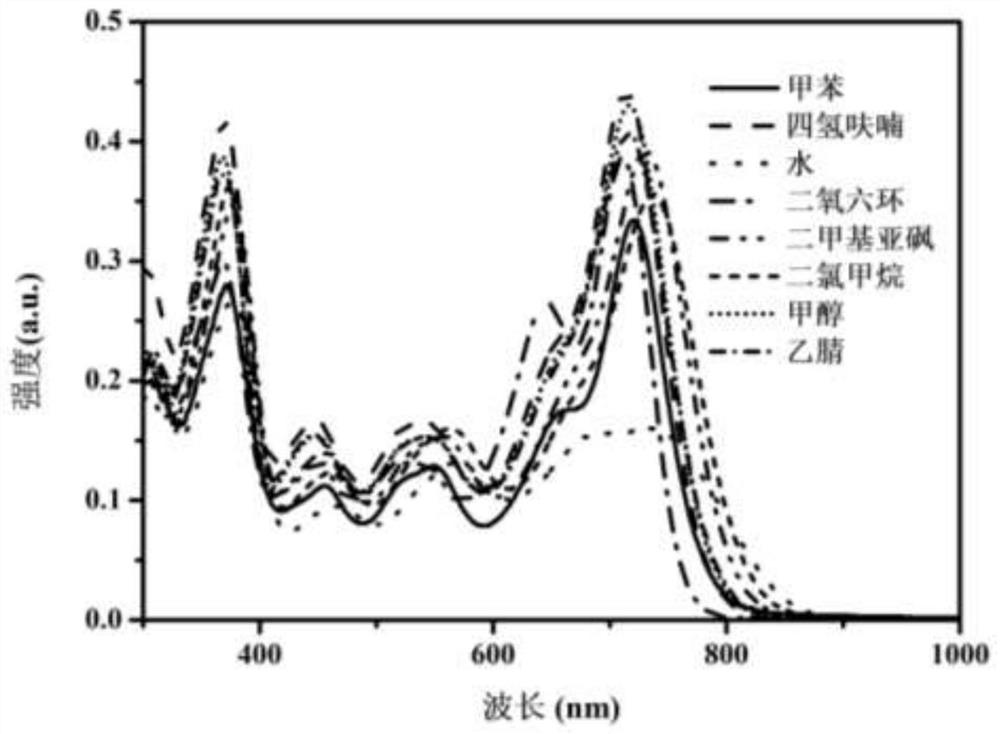
Fluorine-boron dye molecule with donor and acceptor, and quaternary ammonium salt and preparation method thereof
A technology of donating acceptors and quaternary ammonium salts, which is applied in the field of fluoroboron dye molecules and their quaternary ammonium salts and preparations, which can solve the problems of limiting photothermal properties and short emission wavelengths, and improve water solubility and photothermal conversion Efficiency, long absorption wavelength, and good photothermal properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The room temperature in this example refers to 25°C.
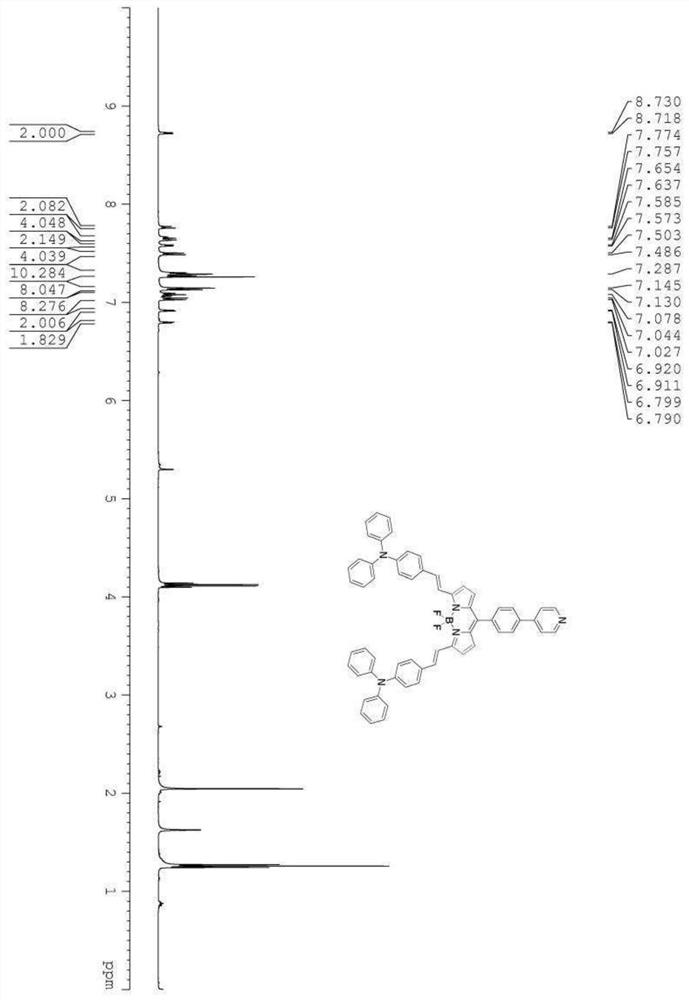
[0054]The preparation method of the donor acceptor fluorine boron dye molecule of formula (I) structure:
[0055] (1) Weigh 1.50g of 4-bromobenzaldehyde and 1.20g of pyridine-4-boronic acid and dissolve them in 20mL of 1,4-dioxane. 4.04 g of potassium carbonate was dissolved in 10 mL of pure water and added to the reaction system. The oxygen in the system was exhausted, and then 0.47 g of tetrakis(triphenylphosphine) palladium was added, and under the protection of nitrogen, the mixture was heated to reflux at 110° C. for 24 h. After the reaction, the 1,4-dioxane solvent was removed by a rotary evaporator, and then purified by a silica gel column to obtain 1.07 g of pyridine-4-carbaldehyde compound.
[0056] (2) Weigh 1.00g of pyridine-4-carbaldehyde compound and 0.88g of 2-methylpyrrole and dissolve in 120mL of dry dichloromethane, add 2 drops of trifluoroacetic acid, and react at room temperature for 12h under t...
Embodiment 2
[0061] The room temperature in this example refers to 22°C.
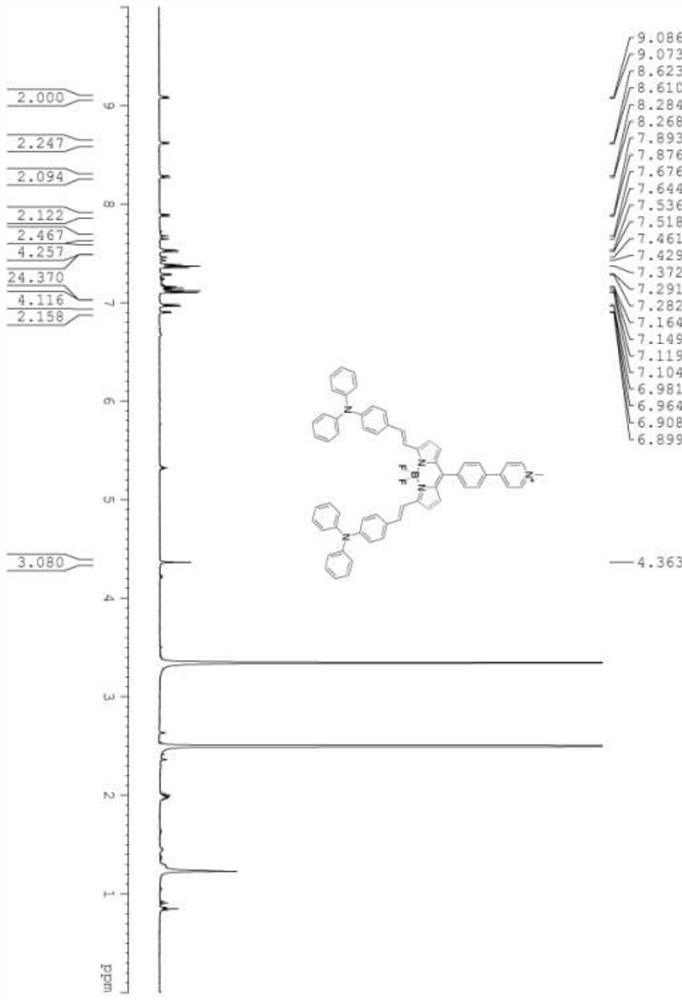
[0062] The preparation method of the donor acceptor fluorine boron dye molecule of formula (I) structure:
[0063] (1) Weigh 2.00g of 4-bromobenzaldehyde and 2.00g of pyridine-4-boronic acid and dissolve them in 25mL of 1,4-dioxane. 6.00 g of potassium carbonate was dissolved in 12 mL of pure water and added to the reaction system. The oxygen in the system was exhausted, and then 0.60 g of tetrakis(triphenylphosphine)palladium was added, and under the protection of nitrogen, the mixture was heated to reflux at 110° C. for 28 h. After the reaction, the 1,4-dioxane solvent was removed by a rotary evaporator, and then purified by a silica gel column to obtain 1.47 g of pyridine-4-carbaldehyde compound.
[0064] (2) Weigh 1.20g of pyridine-4-carbaldehyde compound and 1.05g of 2,4-dimethylpyrrole and dissolve it in 150mL of dry dichloromethane, add a drop of trifluoroacetic acid, and react at room temperature for 12h u...
Embodiment 3
[0069] The preparation method of the donor-acceptor fluoroboron dye molecule of formula (II) structure:
[0070] Weigh 0.04g of the fluoroboron dye molecule obtained in Example 1 and dissolve it in 10mL of toluene, add 0.35mL of methyl iodide (about 0.798g) under the condition of avoiding light, and heat the reaction at 60°C under the protection of nitrogen After 24 hours, no raw materials were spotted on the plate, and the solvent was spin-dried to obtain 43 mg of quaternary ammonium salt with fluoroboron dye molecules for acceptors, denoted as: BDP.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap