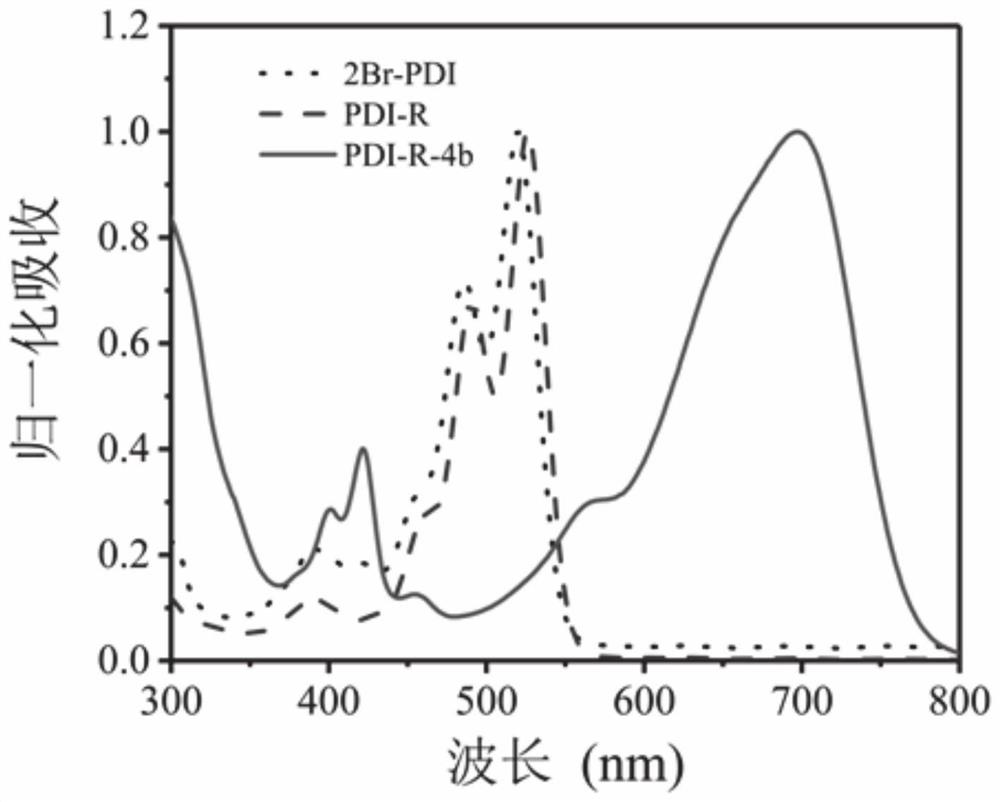
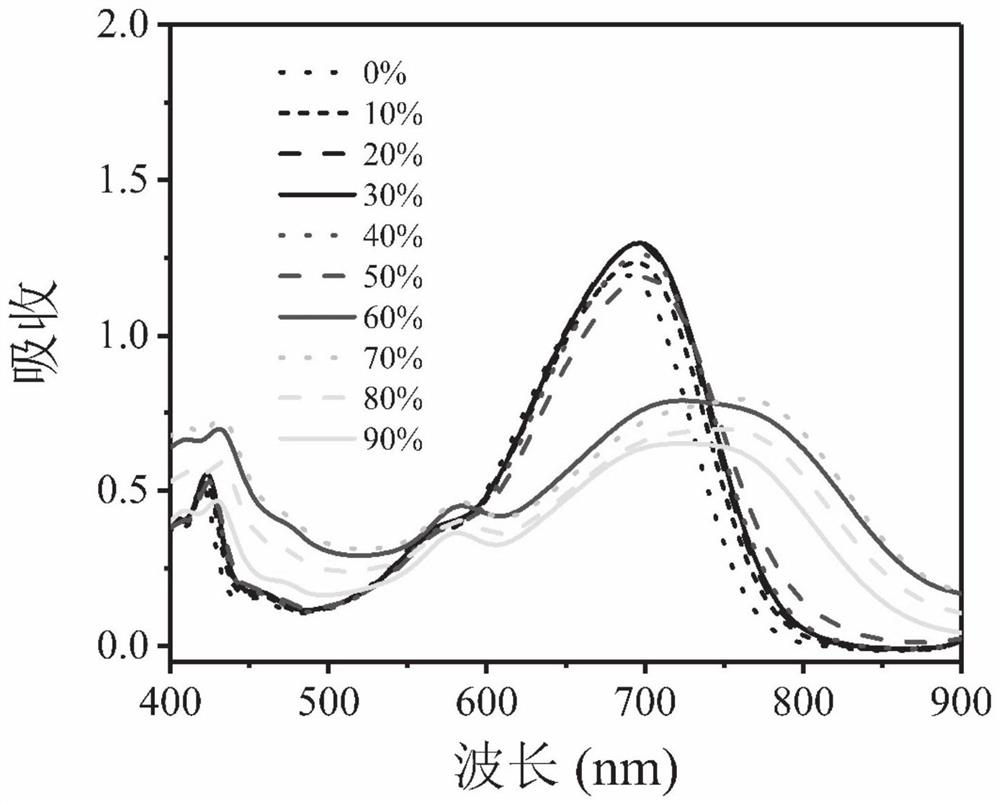
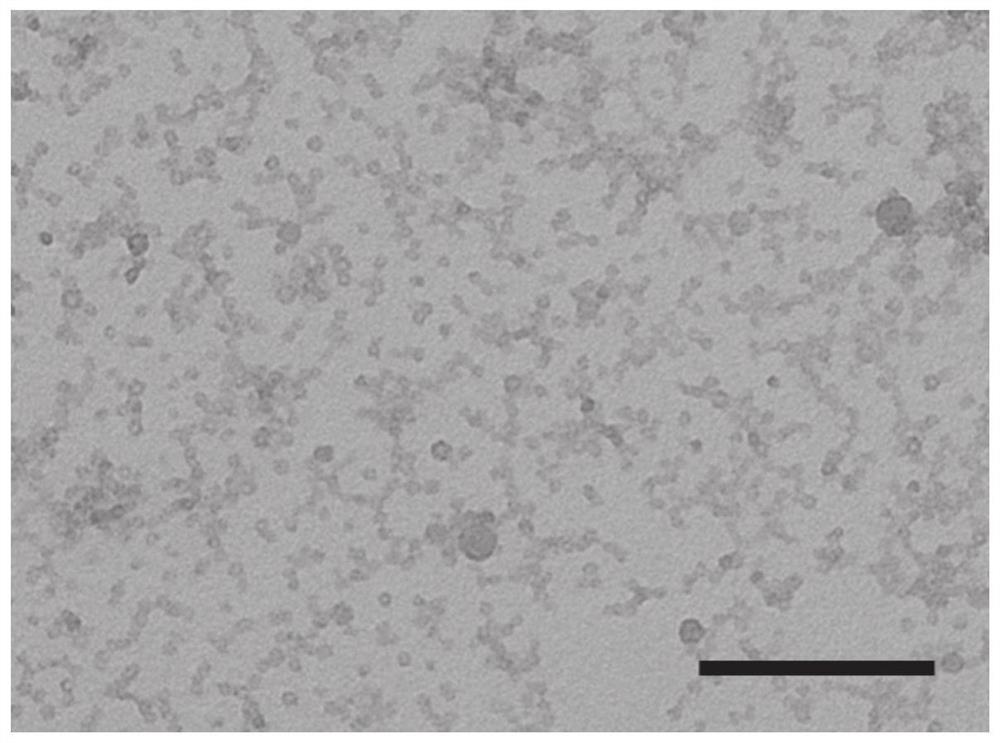
Perylene diimide derivative, hollow nanocapsule PDI-R-4b@SNC as well as preparation method and application of hollow nanocapsule PDI-R-4b@SNC
A perylene diimide and PDI-R technology, applied in the field of photodiagnosis and treatment, can solve the problems of few types and low photothermal conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of perylene diimide derivative PDI-R-4b:
[0049] Synthesis of N,N′-bis((R)-(+)-1-phenethylamino)-1,7-dibromo-3,4,9,10-perylenediimide (PDI-R):
[0050] Dissolve 1,7-dibromo-3,4,9,10-perylenediimide (1 g, 1.82 mmol) and (R)-(+)-1-phenylethylamine (2.2 g, 18.2 mmol) in 50mL of isopropanol was reacted at 82°C for 18h under nitrogen protection. After cooling, the isopropanol was distilled off under reduced pressure. The obtained solid was purified by silica gel column chromatography (dichloromethane:n-hexane=2:1) to obtain pure dark red solid PDI-R with a yield of 46.03%. The specific data of the NMR spectrum of the prepared PDI-R are: 1 H NMR (300MHz, CDCl 3 ,δ):9.45(d,2H),8.89(s,2H),8.65(d,2H),7.50(d,4H),7.36-7.23(m,6H),6.50(m,2H),2.00( d,6H).
[0051] Synthesis of N,N′-bis((R)-(+)-1-phenethylamino)-1,7-dicyclohexylamino-3,4,9,10-perylenediimide (PDI- R-4b):
[0052] PDI-R (300 mg, 0.4 mmol) was dissolved in 15 mL of cyclohexylamine, and the mixture ...
Embodiment 2
[0057] Preparation of perylene diimide derivative PDI-R-4b:
[0058] Synthesis of N,N′-bis((R)-(+)-1-phenethylamino)-1,7-dibromo-3,4,9,10-perylenediimide (PDI-R):
[0059] 1,7-dibromo-3,4,9,10-perylenediimide (0.6g, 1.09mmol) and (R)-(+)-1-phenylethylamine (1.32g, 10.92mmol) were dissolved React in 50 mL of isopropanol at 82°C for 18 h under nitrogen protection, and distill off isopropanol under reduced pressure after cooling. The obtained solid was purified by silica gel column chromatography (dichloromethane:n-hexane=2:1) to obtain pure dark red solid PDI-R with a yield of 52.26%.
[0060] Synthesis of N,N′-bis((R)-(+)-1-phenethylamino)-1,7-dicyclohexylamino-3,4,9,10-perylenediimide (PDI- R-4b):
[0061] PDI-R (150 mg, 0.2 mmol) was dissolved in 15 mL of cyclohexylamine, and the mixture was reacted at 110° C. for 12 h under nitrogen protection. After cooling to room temperature, cyclohexylamine was distilled off under reduced pressure. The obtained solid was purified ...
Embodiment 3
[0063] Preparation of PDI-R-4b@SNC
[0064] Preparation of hollow nanocapsules PDI-R-4b@SNC of polymer and silica-wrapped PDI-R-4b:
[0065] Prepare four small vials, weigh F127 (75mg), then add THF (1mL), ultrasonically dissolve for 5 minutes to obtain a clear and transparent solution, then weigh 5mg of PDI-R-4b and add them to each vial, ultrasonically dissolve, and cap tightly Cover to ensure that the solvent does not evaporate, and stir at 200rpm for 3h; then add TMOS (52μL) and TMVS (13μL) to THF, sonicate, and let stand for use. Then prepare a 20mL vial, weigh 10g of deionized water, and slowly inject 1mL of the organic phase into the deionized water with a syringe under high-speed stirring at 1100rpm, leave it open to allow the organic solvent to volatilize naturally, and stir at 990rpm for 4 days; after the reaction The solution was dialyzed with a dialysis membrane to remove unreacted F127, washed with water for 1 day, and changed water 2-3 times; finally, the obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com