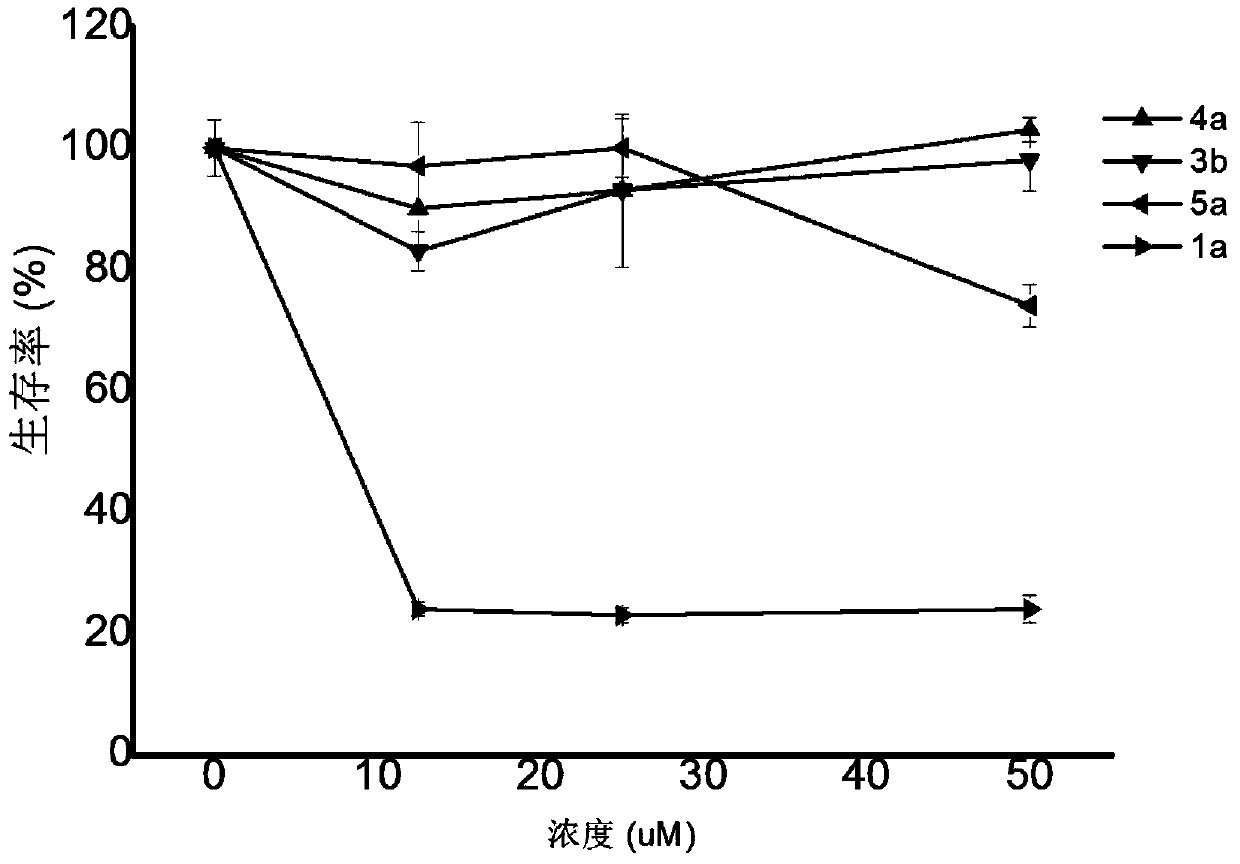
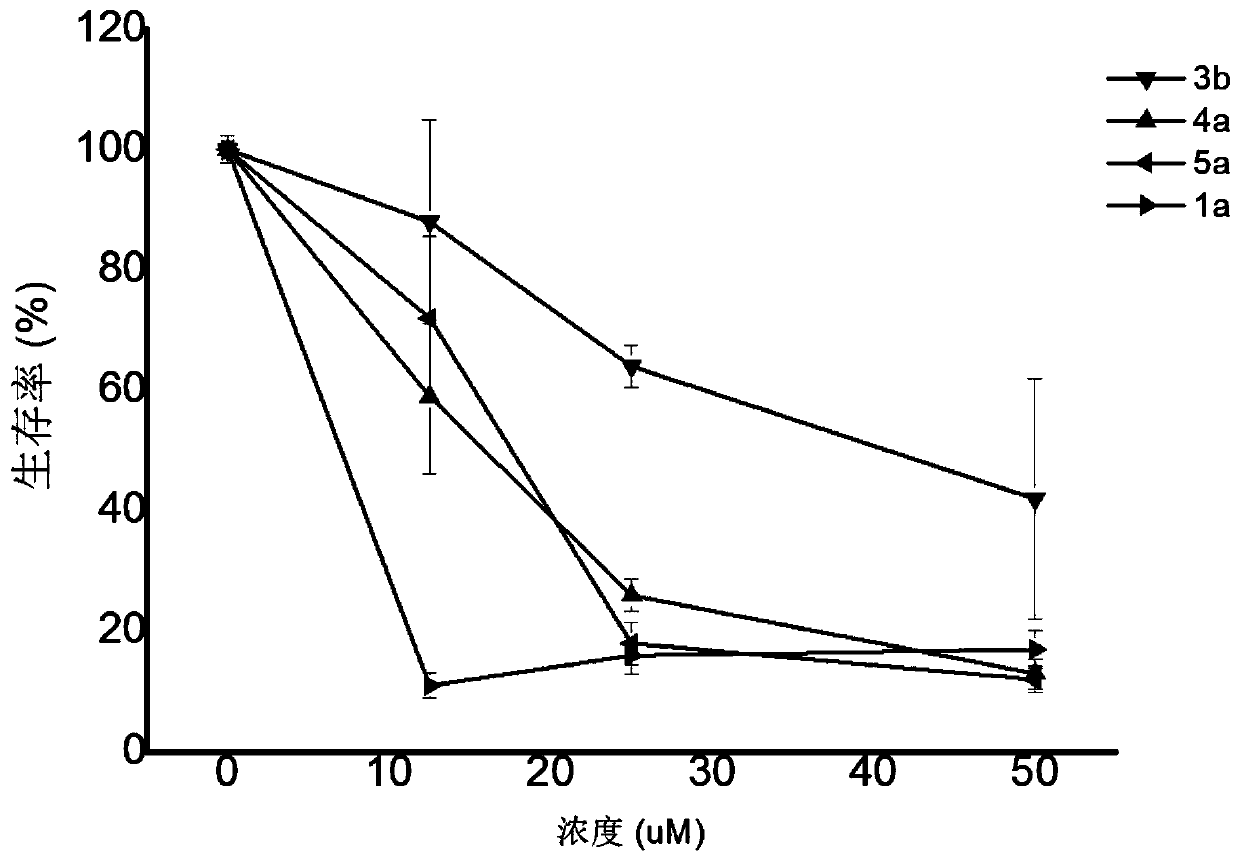
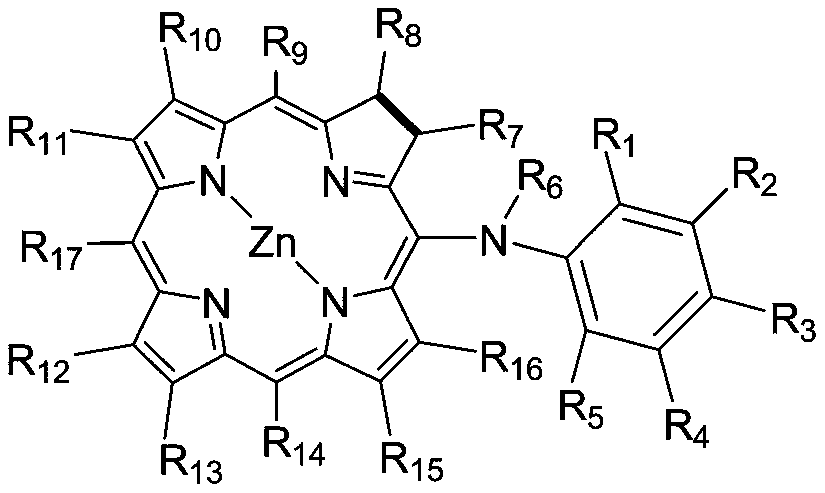
Dihydroporphyrin derivatives and preparation method and application
A technology of chlorin and its derivatives, which is applied in the field of medicine, can solve problems such as limiting the structure and derivatization of chlorin, and achieve the effects of cheap and easy-to-obtain catalysts, high fluorescence quantum efficiency, and long absorption wavelengths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] Take a 50mL round bottom flask, dissolve 0.05mmol of 5,15-di-p-methylphenyl-2,3-dihydrozinc porphyrin (1a) and 0.27mmol of p-toluidine (1a) in freshly distilled In 30mL of dichloromethane solvent, place the round-bottomed flask at room temperature and stir, add NaAuCl to the reaction vessel 4 2H 2 O (0.08mmol), react for about 1min, until the reaction of 1a is complete, quench the reaction with a solution of sodium borohydride (0.26mmol) in methanol (2mL), stir at room temperature for half an hour, then extract with water and dichloromethane, until The aqueous layer becomes colorless, the organic phases are combined, dried with anhydrous potassium carbonate, filtered, rotary evaporated, and the solvent is removed to obtain a mixture containing 10, 10' bilateral amination, 20, 20' directly coupled compound 3a, and then The product 3a was separated and purified by column chromatography with a yield of 68%.
[0050] 1 H NMR (600MHz, CDCl 3 )δ8.85(d, J=4.8Hz...
Embodiment 2
[0052]
[0053] 1a and 2b were used as reactants, and the rest were the same as in Example 1 to obtain the product 10, the 20-position double-sided amination of 5a, with a yield of 60%.
[0054] 1 H NMR (600MHz, CDCl 3 )δ8.77(d, J=4.2Hz, 1H), 8.58(d, J=4.2Hz, 1H), 8.50(d, J=4.2Hz, 1H), 8.36(d, J=4.2Hz, 1H) ,8.31(d,J=4.2Hz,1H),8.02(d,J=4.2Hz,1H),7.89(d,J=7.2Hz,2H),7.67(d,J=5.4Hz,2H),7.44 (d,J=7.2Hz,4H),6.94(s,4H),6.62(s,4H),4.26(m,1H),4.12(m,3H),4.05(s,3H),3.82(s, 3H), 2.62(s,3H), 2.60(s,3H), 2.23(s,3H), 2.21(s,3H)ppm; HR-MS (MALDI) calculated value C 50 h 44 N 6 Zn[M]+792.2919, the actual value is 792.2918.
Embodiment 3
[0056]
[0057] 1a and 2c were used as reactants, and the rest were the same as in Example 1 to obtain product 5b with a yield of 45%.
[0058] 1 H NMR (600MHz, CDCl 3 )δ8.76(d, J=4.2Hz, 1H), 8.56(d, J=4.8Hz, 1H), 8.48(d, J=4.8Hz, 1H), 8.34(d, J=4.2Hz, 1H) ,8.29(d,J=4.2Hz,1H),8.01(d,J=4.8Hz,1H),7.88(m,2H),7.66(m,2H),7.43(m,4H),6.59(m, 6H),6.49(m,2H),4.21(m,1H),4.09(m,3H),4.03(s,3H),3.78(s,3H),3.56(s,3H),3.50(s,3H ), 2.62(s,3H), 2.58(s,3H)ppm; HR-MS(MALDI) calculated value [M]+C 50 h 44 N 6 o 2 Zn 824.2817, theoretical value 824.2818.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com