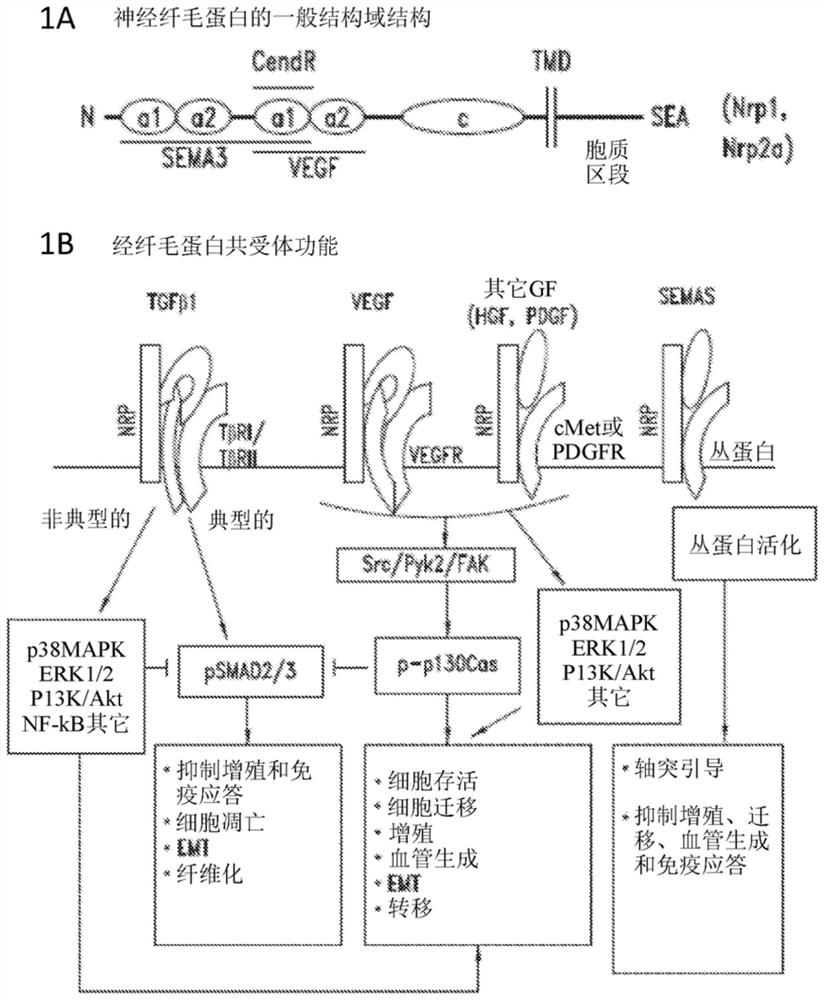
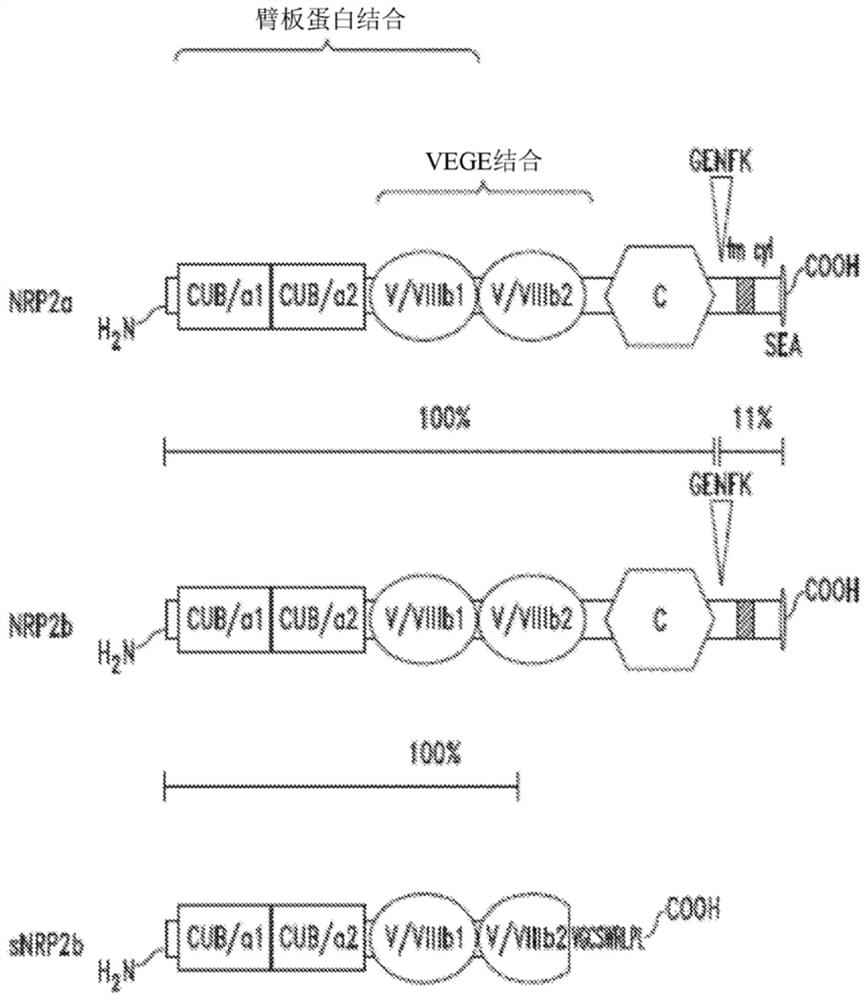
Compositions and methods for treating nrp2-associated diseases
A technology for treating compositions and diseases, applied in the field of compositions and methods for treating NRP2-related diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0682] Materials and methods
[0683] ELISA assay
[0684] Assays for measuring circulating human and mouse endogenous HRS An ELISA detection assay was developed to detect circulating small The levels of murine or human HRS were quantified.
[0685] The human N-terminal ELISA was designed to detect the N-terminal domain of human HRS (WHEP domain) using capture and detection antibodies that target this domain (approximately amino acids 1-60 of HRS).
[0686] Using 96-well multi-array plates coated with capture antibodies, the ELISA assay was performed following a standard Meso Scale Diagnostics ELISA protocol and using the following reagents:
[0687] · Blocking buffer: Casein (Thermo Scientific #37528)
[0688] Wash buffer: PBST (1X PBS with 0.05% Tween-20; prepared in-house)
[0689] · Diluent: 1% BSA (diluted in PBS) and casein
[0690] Capture antibody: ATYR12H6, mouse monoclonal antibody
[0691] ·Capture antibody concentration: 1μg / mL
[0692] ·Protein standard ran...
example 1
[0826] Initial Receptor Identification Screening
[0827]To identify potential interacting partners of HRS and related HRS polypeptides, HRS-Fc fusion protein constructs were evaluated using Retrogenix cell microarray screening technology (Retrogenix Ltd., High Peak Rd, United Kingdom) ( Binding of [Fc-HRS(2-60)] to a library of approximately 4500 membrane-bound human proteins expressed individually in HEK293 cells.
[0828] Briefly, HEK293 cells were plated on glass coverslips that had been pretreated by application of discrete expression vectors to enable reverse transfection and expression of each of the 4500 membrane proteins Seed membrane proteins to generate cellular microarrays. Transfection efficiency was assessed by ZsGreen1 expression and exceeded the minimum threshold for all library members screened.
[0829] Using an Fc-tagged smaller HRS fragment provides highly sensitive detection by using an AlexaFluor 647-labeled anti-human IgG Fc antibody (AF647) as a detec...
example 2
[0838] Confirmation of binding specificity by SPR analysis and identification and use of specific epitopes
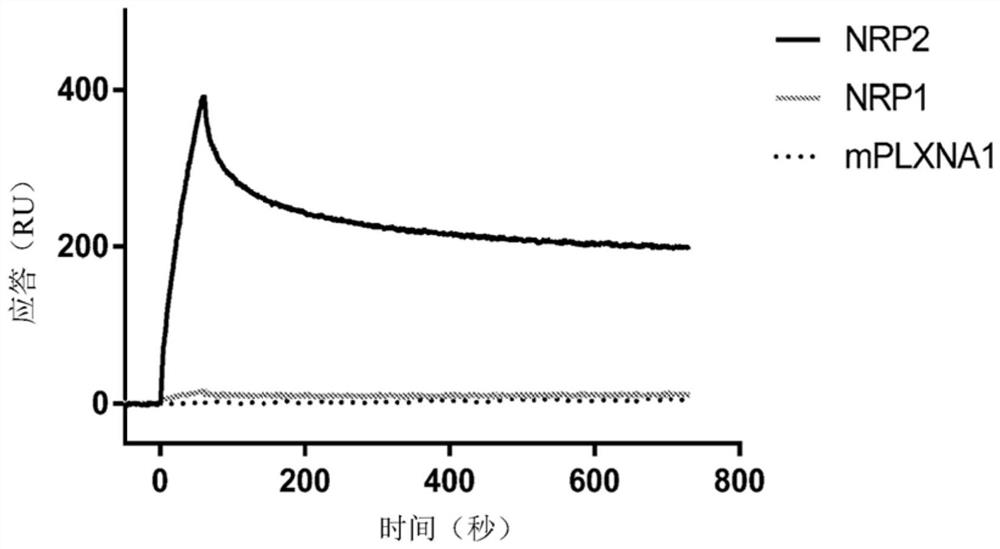
[0839] A study was performed to confirm the binding specificity of Neuropilin 2 (NRP2) to Fc-HRS(2-60) using an orthologous approach to that used in the large-scale Retrogenix screen (Example 1). In a series of experiments, Fc-HRS(2-60) and related proteins were immobilized on an SPR chip, and NRP2 and related proteins were flowed as analytes. After confirming the NRP2:Fc-HRS(2-60) interaction, the dependence on divalent cations was tested since NRP2 is known to have Ca 2+ binding site. The effect of previously characterized NRP2 ligands on the NRP2:Fc-HRS(2-60) interaction was also tested to determine whether these known ligands compete for the Fc-HRS(2-60) interaction.
[0840] In another series of experiments, a monoclonal antibody (mAb) recognizing Fc-HRS(2-60) was immobilized on an SPR chip. Fc-HRS(2-60) and NRP2 were pre-incubated and injected onto the mAb surf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com