Method for determining the in vitro dissolution of felodipine sustained-release tablets
A felodipine and gentle technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that cannot truly reflect the dissolution behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
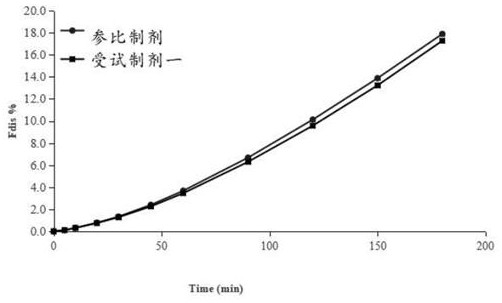
[0043] A method for measuring the in vitro dissolution of felodipine sustained-release tablets, the felodipine sustained-release tablets are dissolved using a two-chamber model experimental device that simulates the in vivo dissolution and transmembrane absorption process of insoluble oral pharmaceutical preparations, and the specific steps are:
[0044] 1) The dissolution medium enters the porous membrane cup through the action of the inlet pump. When the volume of the dissolution medium in the porous membrane cup and the outer chamber dissolution cup is the same, the outlet pump is turned on. The working frequency of the inlet pump and the outlet pump are consistent, keeping The total volume of the dissolution medium remains unchanged; the dissolution medium is pH 6.8 phosphate buffer with 0.3% sodium lauryl sulfate, and the pore size of the filter membrane coated on the porous filter cup is 0.45 μm;
[0045] 2) Put Felodipine Sustained-release Tablets in the rotating basket,...
Embodiment 2
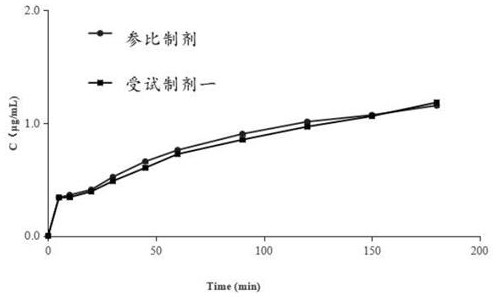
[0053] A randomized, open, three-period, cross-over experimental design was used to conduct a bioequivalence study on 12 healthy volunteers before meals. Preparation or test preparation 5mg / time, once a day, blood collection time is designed as before administration (0h) and after administration 10min, 20min, 30min, 45min, 1.0h, 1.25h, 1.5h, 1.75h, 2h, At 2.5h, 3h, 4h, 5h, 6h, 8h, 10h, 12h, 15h, 24h, and 36h, 4 mL of upper limb venous blood was collected respectively. The samples were centrifuged (3,500 rpm, 10 min) to separate the plasma and stored in a -80°C ultra-low temperature freezer. Specifically, take 100 μL of plasma sample, add 600 μL of anhydrous methanol (including internal standard), vortex for 5 minutes, mix well, centrifuge at 12,000 rpm for 10 minutes, take the supernatant and analyze the non- Lodipine content.
[0054] The conclusion is shown in Table 1:
[0055] Table 1 Summary of main pharmacokinetic parameters of fasting felodipine
[0056]
[0057] ...
Embodiment 3
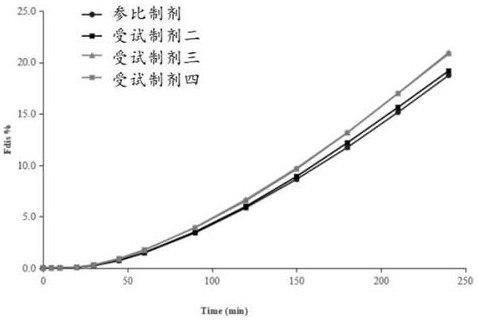
[0061] A method for measuring the in vitro dissolution of felodipine sustained-release tablets, the felodipine sustained-release tablets are dissolved using a two-chamber model experimental device that simulates the in vivo dissolution and transmembrane absorption process of insoluble oral pharmaceutical preparations, and the specific steps are:
[0062] 1) The dissolution medium enters the porous membrane cup through the action of the inlet pump. When the volume of the dissolution medium in the porous membrane cup and the outer chamber dissolution cup is the same, the outlet pump is turned on. The working frequency of the inlet pump and the outlet pump are consistent, keeping The total volume of the dissolution medium remains unchanged; the dissolution medium is pH 6.8 phosphate buffer with 0.3% sodium lauryl sulfate, and the pore size of the filter membrane coated on the porous filter cup is 0.45 μm;
[0063] 2) Put Felodipine Sustained-release Tablets in the rotating basket,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com