Compositions and methods for treating leber's hereditary optic neuropathy
A technology for compositions and pharmaceutical compounds, which can be applied in the fields of botanical equipment and methods, biochemical equipment and methods, and introduction of foreign genetic material using vectors, which can solve problems such as the reduction of mitochondrial NADH dehydrogenase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0212] Preparation of carrier
[0213] The carrier of the present invention can be prepared by a standard method known in the art for providing a vector of gene therapy. Therefore, mature common domain transfections, packaging and purification methods can be used to prepare suitable carrier preparations.
[0214] As discussed above, in addition to the polynucleotide sequence encoding ND4, ND6 or ND1 or variants, the vector of the present invention may further comprise a genome of naturally occurring AAV virus. However, a derivatized genome will be used, for example, having at least one end reverse repeat sequence (ITR) but may lack any AAV gene such as REP or CAP.
[0215] In such an embodiment, in order to assemble the derivatization gene into AAV viral particles, additional genetic constructs providing AAV and / or auxiliary viral function will be provided in the host cell to the derivatized genome combination. These additional constructs typically contain genes encoding a genet...
Embodiment 1
[0329]Example 1-ND4 plasmid and virus preparation
[0330] 1.1 plasmid preparation
[0331] Nucleotide sequences of human ND4 (SEQ ID NO: 6) were obtained based on the US National Biotechnology Information Center Reference Sequence YP_003024035.1. The sequence of non-optimized mitochondrial targeting sequence COX10 is SEQ ID NO: 1. The optimized sequence (OPT_COX10, SEQ ID NO: 2) of the mitochondrial targeting sequence COX10 and the coding sequence of the human ND4 (OPT_ND4, SEQ ID NO: 7) to improve transcription efficiency and translation efficiency. After the unopened COX10-ND4 has about 75.89% homology optimized COX10-ND4 sequence, it is the three start-independent districts of recombinant nucleic acid OPT_COX10-OPT_ND4-3'UTR (e.g., SEQ ID NO: 31) (ie , 3'UTR, SEQ ID NO: 13).
[0332] The synthetic recombinant nucleic acid OPT_COX10-OPT_ND4-3'UTR was incorporated into the adenocarid virus (AAV) vector by PCR amplification. figure 1 ). The OPT_COX10-OPT_ND4-3'UTR is cut by Ec...
Embodiment 2
[0342] Example 2 - Raav2 in the rabbit eye glass
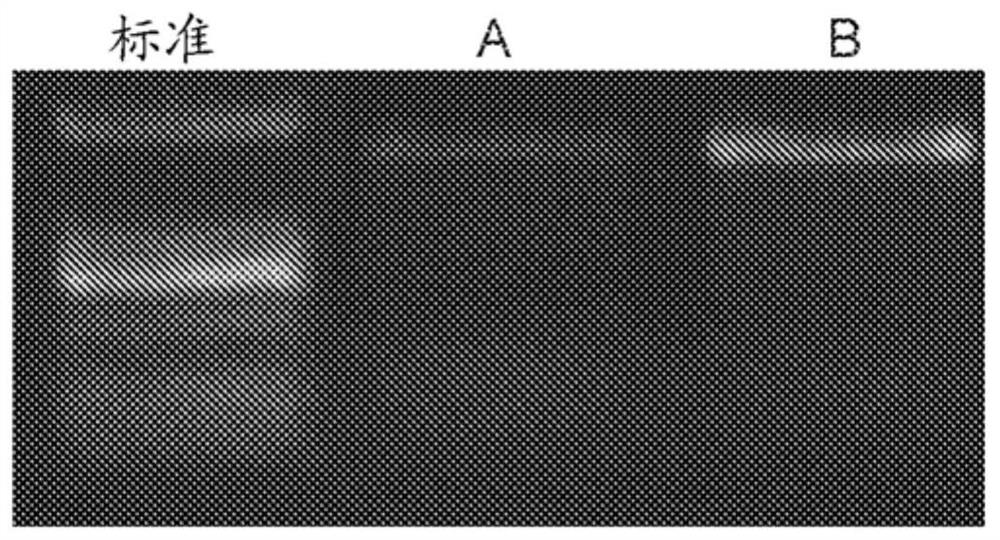
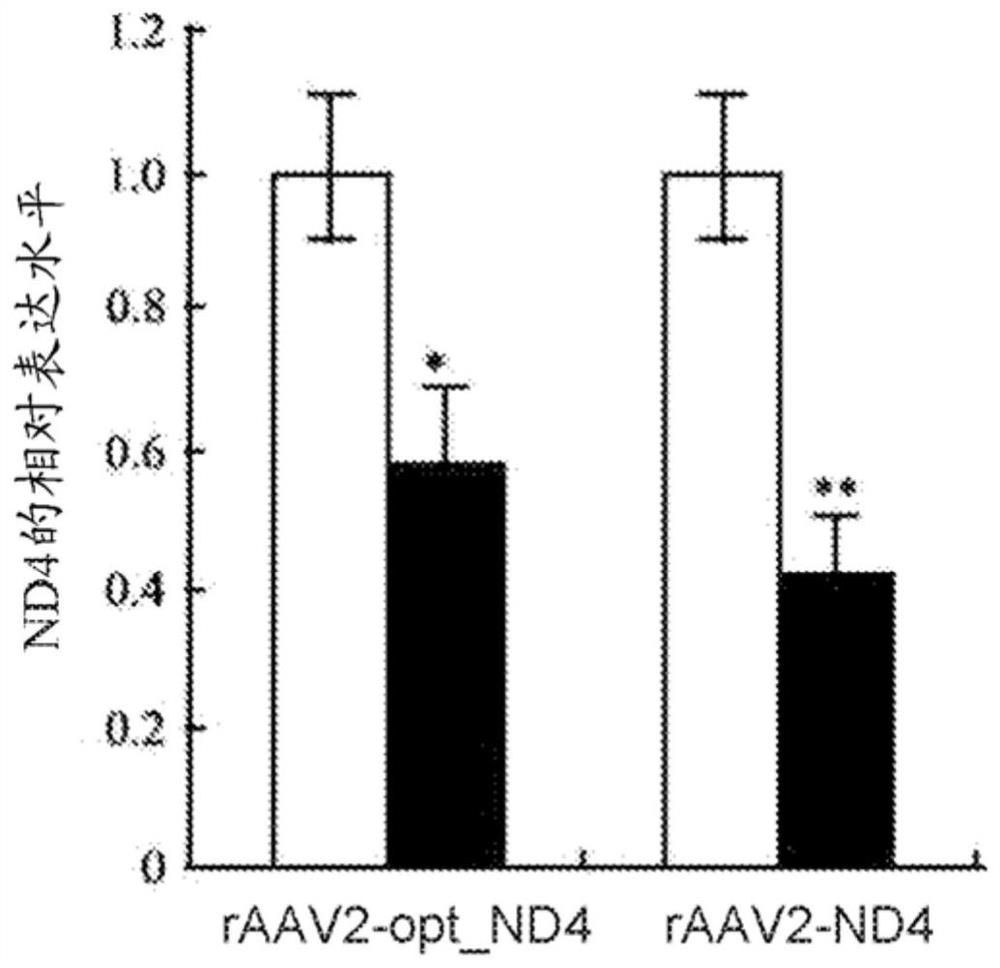
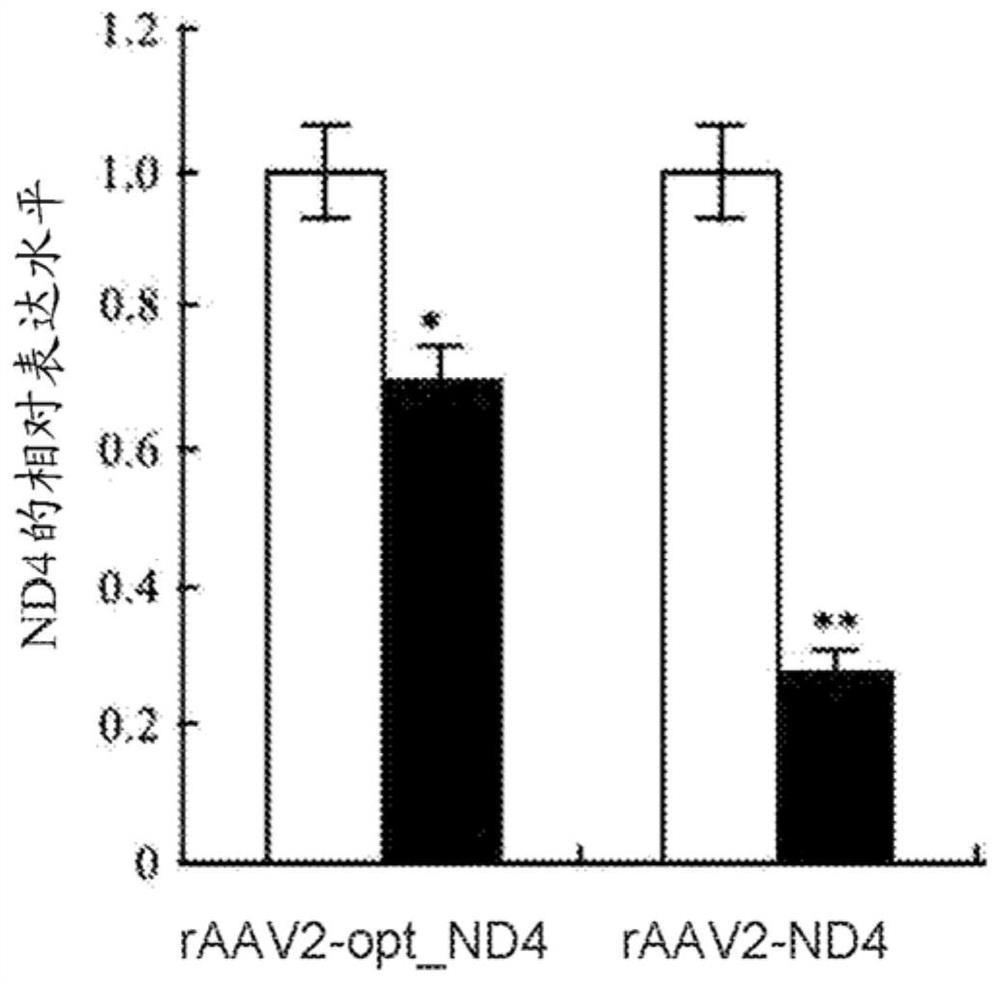
[0343] Twelve rabbits were divided into 2 groups: RAAV2-ND4 and RAAV2-optimized ND4. The viral solution (1 × 10 10 Vg / 0.05 mL) was pierced into the glass body cavity from 3 mm of the corneal edge of the flat portion. After injection in the glass, the eye is checked by a crack lamp check and the fundus photographic examination. Injection for 30 days. RT-PCR detection and immunoblot were carried out in each group.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com