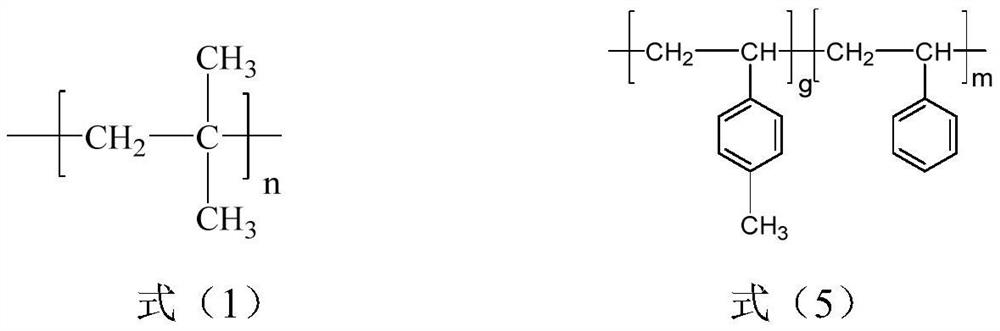Styrenic block copolymer and preparation method thereof
A technology of block copolymers and styrenes, which is applied in the field of styrenic block copolymers and its preparation, can solve the problems of cumbersome steps for hydroxyl-functionalized polymers, difficulty in controlling the sulfonation reaction process, and cumbersome preparation process, etc. Achieve the effects of improving the use temperature and thermal stability, enhancing interaction, and enhancing hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the styrenic block copolymer provided by the invention comprises the following steps:
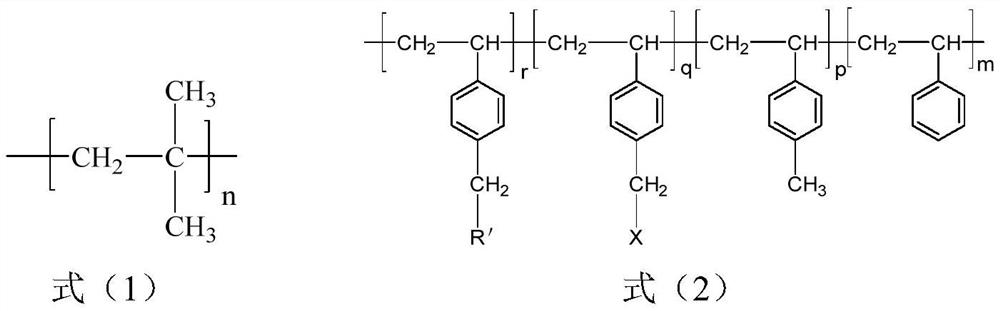
[0028] S1, isobutylene, styrene and p-methylstyrene are obtained through sequential living cationic copolymerization in the presence of an initiating system to obtain a polyisobutylene segment with a structure shown in formula (1) and styrene with a structure shown in formula (5) Block copolymers (SMB) of random copolymerized segments with p-methylstyrene structural units;
[0029]
[0030] Wherein, n is 200~1800, the total number (m) of styrene structural units accounts for 0.5~29% of the total moles of all structural units, and the total number (g) of p-methylstyrene structural units accounts for 0.5~29% of the total moles of all structural units. 1.05-43%.
[0031] S2, carry out bromination reaction / chlorination reaction in organic solvent with the block copolymer obtained in step S1 and bromination / chlorination reagent, obtain bromination / chlorinatio...
Embodiment 1
[0078] This example serves to illustrate the azonia-ionized poly[(p-methylstyrene-co-styrene)-b-isobutylene-b-(styrene-co-p-methylstyrene)] block copolymer (FSMB -1) Preparation and properties.
[0079] Preparation of S1 and SMB-1
[0080] Under the protection of high-purity nitrogen at -78°C, 110 mL of dichloromethane, 164 mL of n-hexane, 0.3 mol of isobutene (IB) and 0.845 mmol of bifunctional initiator 1,4-bis(2-chloro-2 -Propyl)benzene (DCC), add 7.0mL containing co-initiator FeCl 3 With the additive isopropanol (POH) solution (of which: FeCl 3 :POH=1:1.4), so that the concentration of IB in the reaction system is 1.0mol / L, DCC:FeCl 3 : IB=1:3.6:355 (molar ratio), reaction 15min, IB monomer is fully converted, obtains polyisobutylene double-end active chain; Add 100mL containing styrene (St, 68.7mmol) and p-methylstyrene (pMS, 34.4mmol) in dichloromethane / normal hexane (4 / 6, v / v) mixed solution, further carry out active cationic block copolymerization reaction, the rea...
Embodiment 2
[0088] The preparation method of S1 and SMB-2 is the same as step S1 in Example 1. At -80°C and under the protection of high-purity nitrogen, add 220mL of dichloromethane, 328mL of n-hexane, 0.6mol of isobutene and 3.38mmol of DCC into the polymerization reactor and mix well, add 14mL of FeCl containing co-initiator 3 With POH solution (where: FeCl 3 :POH=1:1.4), so that the concentration of IB in the reaction system is 1.0mol / L, DCC:FeCl 3 : IB=1:3.6:178 (molar ratio), polymerization reaction 15min, IB monomer is fully transformed, obtains polyisobutylene two-terminal active chain; Only the feeding amount of St and pMS mixed solution is reduced, add 120mL containing 82.4mmol styrene and 41.2mmol methylene chloride / n-hexane (4 / 6, v / v) mixed solution of p-methylstyrene, obtain poly(styrene-co-p-methylstyrene)-b-polyisobutylene-b-poly( Styrene-co-p-methylstyrene) triblock copolymer SMB-2.
[0089] S2, BSMB-2 preparation method is the same as step S2 in embodiment 1, just Br2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com