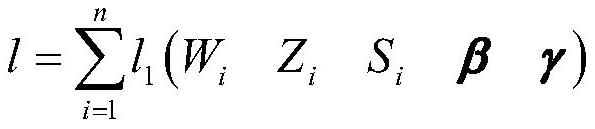Data processing method and system based on clinical test data
A clinical trial and data processing technology, applied in the field of data processing methods and systems based on clinical trial data, can solve problems such as insufficient elimination of population heterogeneity inference accuracy, and achieve the effect of eliminating bias
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
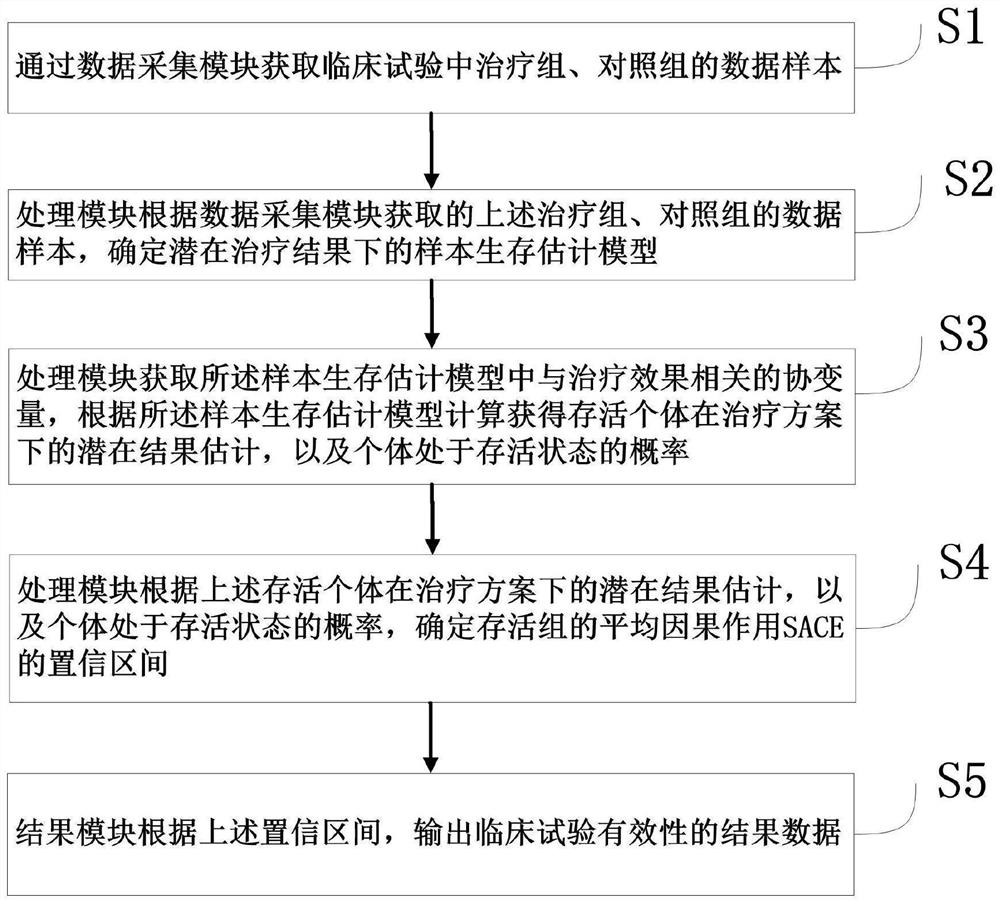
[0086] A specific embodiment of the present invention discloses a data processing method based on clinical trial data, such as figure 1 shown, including the following steps:
[0087] S1. Obtain data samples of the treatment group and control group in the clinical trial.
[0088]Among them, the data sample size of the clinical trial is 2n, and the data sample sizes of the treatment group and the control group are n respectively. The data samples of the treatment group are data samples corresponding to a certain treatment plan (including drugs or treatment means or treatment process, marked as z), and the data samples of the control group are data samples corresponding to only placebo or control measures.
[0089] S2. Based on the above-mentioned data samples of the treatment group and the control group, determine a sample survival estimation model under potential treatment outcomes. The sample survival estimation model includes a potential outcome model for individuals with a...
Embodiment 2
[0096] On the basis of the method in Example 1, the data samples of the treatment group and the control group include covariate set W, treatment status Z, survival status S, and quality of life rating Y related to the treatment effect.
[0097] The treatment status Z is 0 means no treatment, the individual is in the control group, 1 means the treatment of treatment plan z is implemented, and the individual is in the treatment group.
[0098] The survival status S is 0 for dead and 1 for alive.
[0099] Treatment effect Y or quality of life rating Y can be set according to needs, for example, 0 means no impact, 1 means slight impact, and 2 means severe impact.
[0100] Let Y(Z) and S(Z) denote potential outcome and potential survival status at treatment treatment status Z. In fact, in the experiment, only one of S(0) and S(1) can be observed, because only one treatment can be applied to the individual; only when the observed S(Z) is equal to 1, we can Y(Z) of the response is ...
Embodiment 3
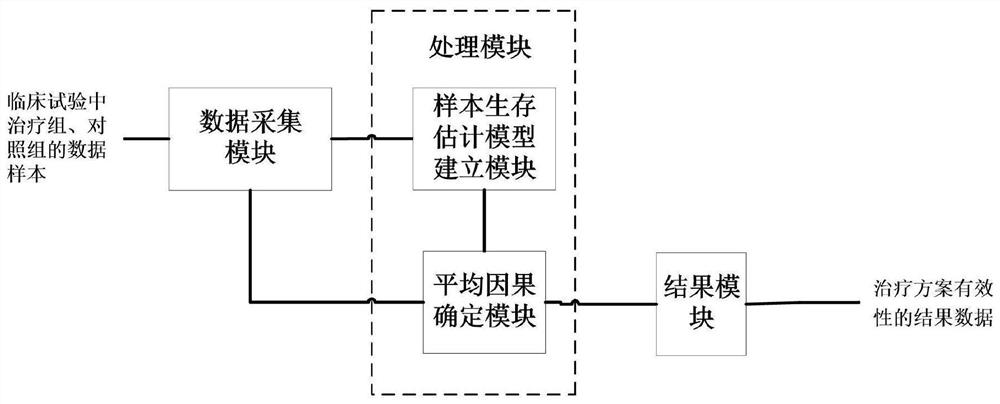
[0186] The present invention also provides a kind of data processing system corresponding to embodiment 1, 2, comprises the data acquisition module, processing module, result module connected in sequence, such as figure 2 shown.
[0187] The data collection module is used to obtain the data samples of the treatment group and the control group in the clinical trial.
[0188] The processing module is used to determine the sample survival estimation model under the potential treatment result based on the data samples of the treatment group and the control group; and obtain the covariates related to the treatment effect in the sample survival estimation model, and according to the sample survival estimation The model calculation obtains the potential outcome estimate of the surviving individual under the treatment plan and the probability of the individual being in the surviving state; and, according to the above-mentioned potential outcome estimate of the surviving individual un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com