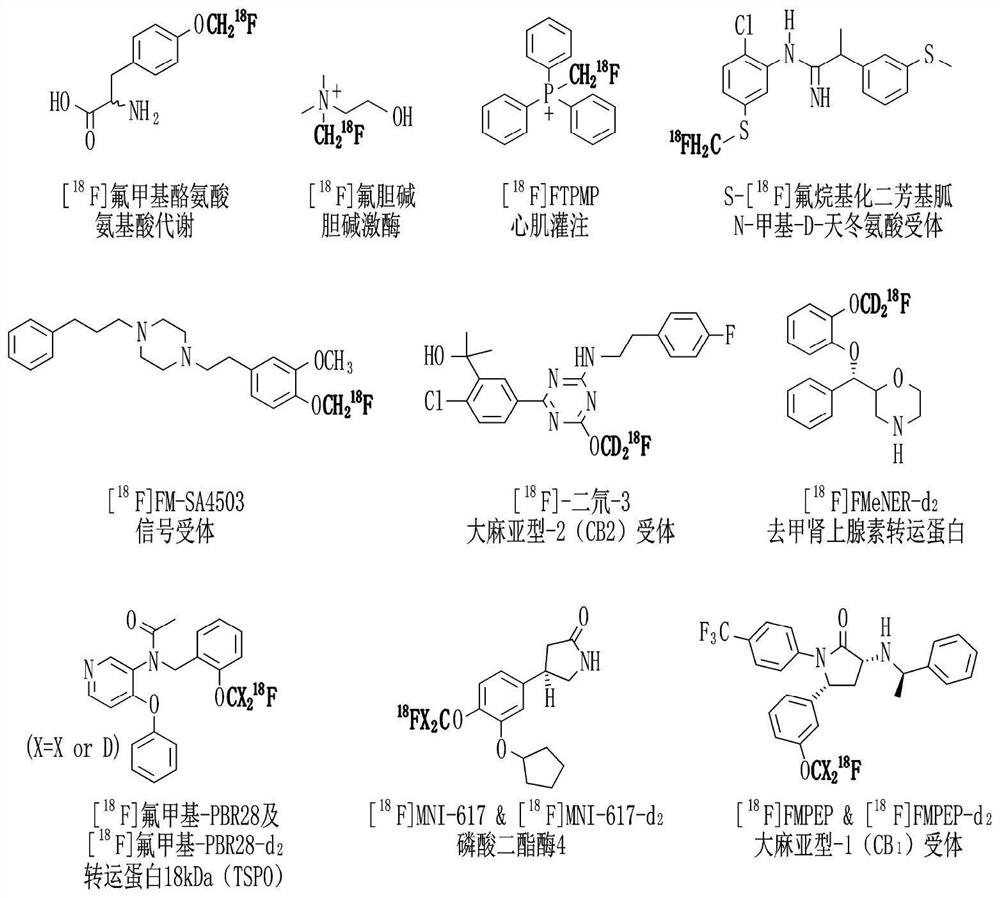
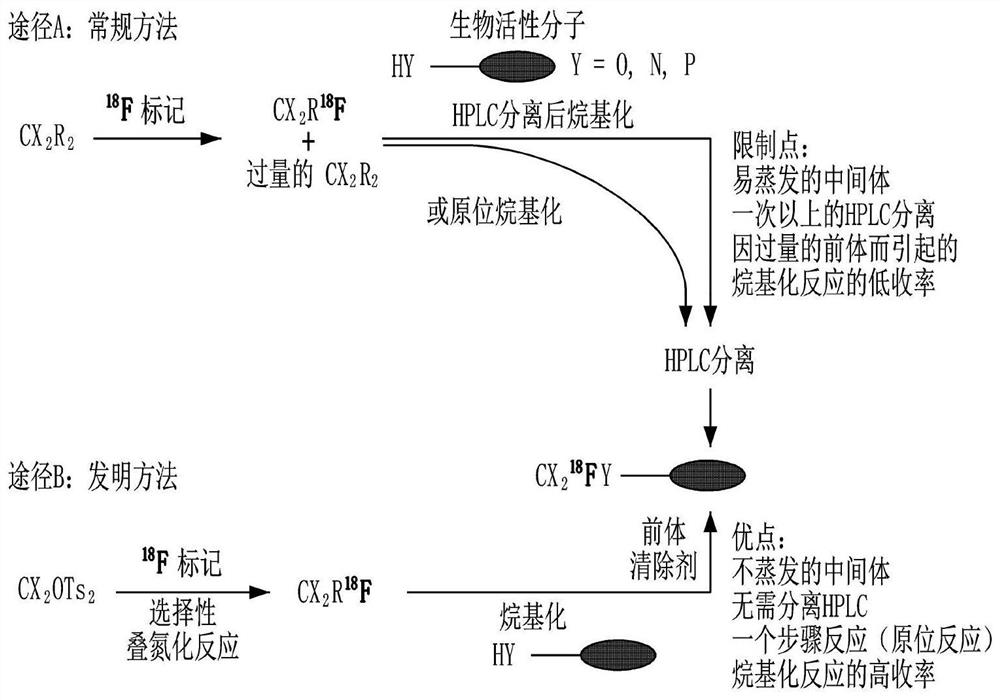
Method for preparing fluorine-18-labeled fluoromethyl-substituted radiopharmaceuticals using selective azide substitution reaction and precursor scavenging
A technology of azide compound and fluoromethyl group is applied in the field of preparation of fluoromethyl-substituted radiopharmaceuticals marked with fluorine-18 by using selective azide compound substitution reaction and precursor scavenging, which can solve the cumbersome and long-term Labeling time, low labeling yield and other problems, to achieve the effect of cost saving, high labeling yield, high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] In order to achieve the above object, the preparation method of the fluorine-18 labeled fluoromethyl-substituted radiopharmaceutical using the selective azide substitution reaction of the present invention comprises: the first step, passing through the cyclotron 18 O(p,n) 18 F reacts to get [ 18 F] fluoride; the second step, using dissolved K 2.2.2 and K 2 CO 3 The acetonitrile reaction solution was separated from the above [ 18 F] Fluoride to get [ 18 F]F - / H 2 18 O solution; the third step, heating the above [ 18 F]F - / H 2 18 O solution to get K 2.2.2 / K 18 F; the fourth step, the above K 2.2.2 / K 18 F and bis(toluenesulfonyloxy)methane compound are put into the reaction vessel together, and the reaction solution is added to react to obtain the first precursor solution; the fifth step is to cool the above-mentioned first precursor solution, add the azide compound, and only Prepare [ 18 F] fluoromethyl tosylate compound; the sixth step, in the above ...
Embodiment 1
[0078] Hereinafter, experiments using F-19 and F-18 in radiopharmaceuticals produced by the method for producing fluoromethyl-substituted radiopharmaceuticals utilizing the selective azide substitution reaction and precursor scavenging of the present invention according to the examples The process is detailed below.
[0079] Experiment 1
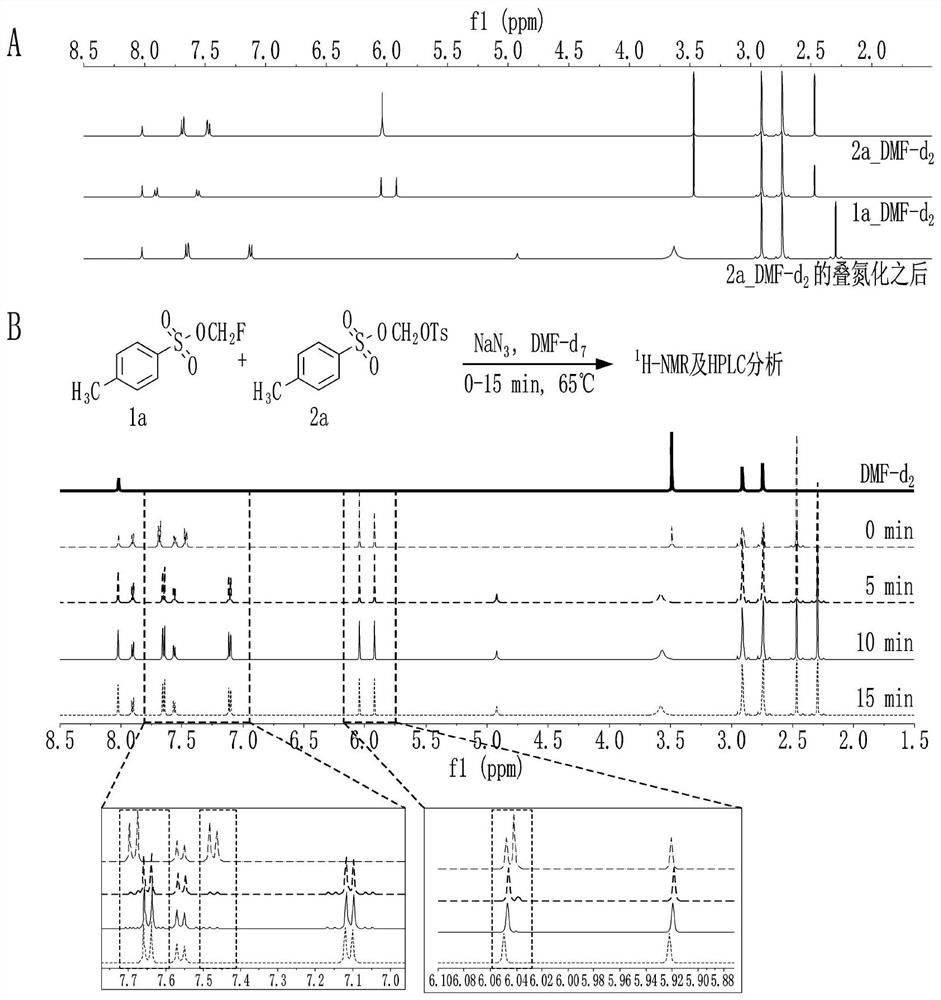
[0080] 1 H-NMR and HPLC analysis
[0081] Dissolve the same equivalent (4.9 mmol) of 1a and 2a in DMF-d as NMR solvent 7 Afterwards, with NaN 3 (2.5 eq., 12.3 mmol) work-up followed by reaction at 65°C. Over time, the results were analyzed by NMR and HPLC at 5 min, 10 min, 15 min intervals compared to the 0 min results for untreated azide. The azide anion (N 3 - ) appears to be a competing reaction between 1a and 2a, more relatively bis(tosyloxy)methane, which is electron-rich at the methyl carbon, preferentially undergoes azide substitution. As predicted, it was confirmed that bis(toluenesulfonyloxy)methane was significantly more ...
preparation example 1
[0101] Preparation of bis(toluenesulfonyloxy)methane compound
[0102]
[0103] After dibromomethane (500 uL, 7.12 mmol) and silver p-toluenesulfonate (4.17 g, 14.95 mmol) were dissolved in acetonitrile (8 mL), it was allowed to reflux for 16 hours. After completion of the reaction, extraction was performed with water and dichloromethane, only the organic solvent layer was separated, and water was removed with sodium sulfate, followed by filtration. Removal of the solvent from the filtered solution afforded 2a-b as white solids.
[0104] Anal. Calculated for (C 15 h 16 o 6 S 2 ,2a): C, 50.55; H, 4.53; O, 26.93; S, 17.99%. MS (ESI) m / z 357.24 (M+H + ); m.p.119.9-122.1℃. 1 H NMR (400MHz, CDCl 3 )δ7.59(d, J=8.4Hz, 3H), 7.24(d, J=8.4Hz, 4H), 5.81(s, 2H), 2.45(s, 6H); 13 C NMR (100MHz, CDCl 3 )δ145.2, 133.1, 129.6, 127.8, 87.79, 21.6.
[0105] Anal. Calculated for (C 15 h 14 D. 2 o 6 S 2 ,2b): C, 50.27; H, 5.06; O, 26.78; S, 17.89%. MS (ESI) m / z 359.24 (M+H) + ;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com