Chiral quaternary ammonium salt phase transfer catalyst based on tetramethyl spirobiindane skeleton and preparation method thereof
A technology of tetramethylspirodihydroindene and phase transfer catalyst, applied in the field of chemistry, can solve problems such as unsatisfactory stereoselectivity, and achieve the effects of easy structure modification and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
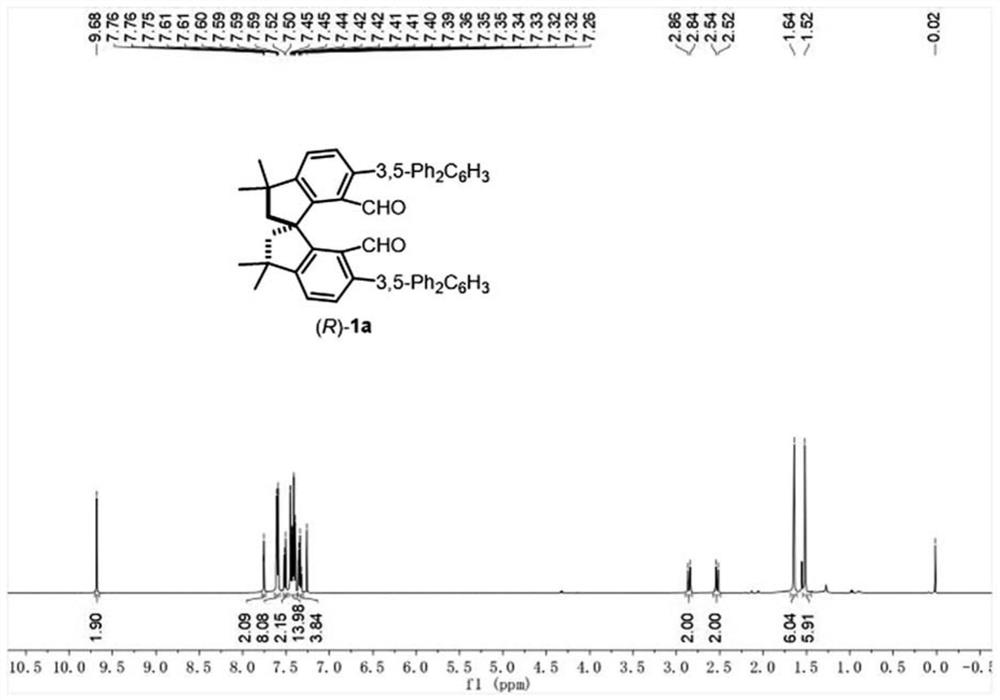
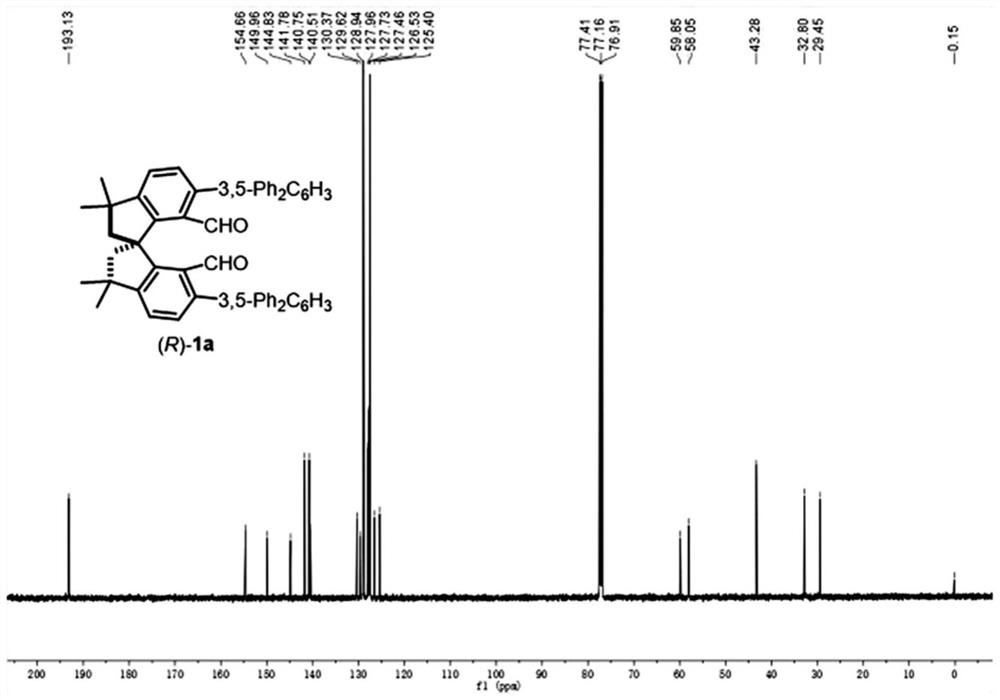
[0054] Under nitrogen protection, (R)-II (1.5g, 2.39mmol), tetrakis(triphenylphosphine) palladium (277.3mg, 0.24mmol), 3,5-diphenylbenzene Boric acid (1.64g, 5.98mmol), anhydrous potassium carbonate (1.32g, 9.56mmol), DMF (55mL), the reaction solution was heated to 70°C and stirred for 8 hours. After the reaction was complete, the reaction solution was cooled to room temperature, the reaction solution was quenched with 10 mL of distilled water, and extracted with EtOAc (150 mL), the organic phase was washed with deionized water (5×100 mL), and then washed with saturated brine (100 mL), Dry over anhydrous sodium sulfate, filter, and remove the organic solvent by rotary evaporation under reduced pressure. The obtained crude product is purified by silica gel column chromatography, and the eluent is ethyl acetate:petroleum ether=1:100-1:50 to obtain (R)-1a It is 1.72g, is a white solid, and the yield is 92%; 1 H NMR (500MHz, CDCl 3 )δ9.68(2H,s),7.76(2H,t,J=10.0Hz),7....
Embodiment 2
[0059]
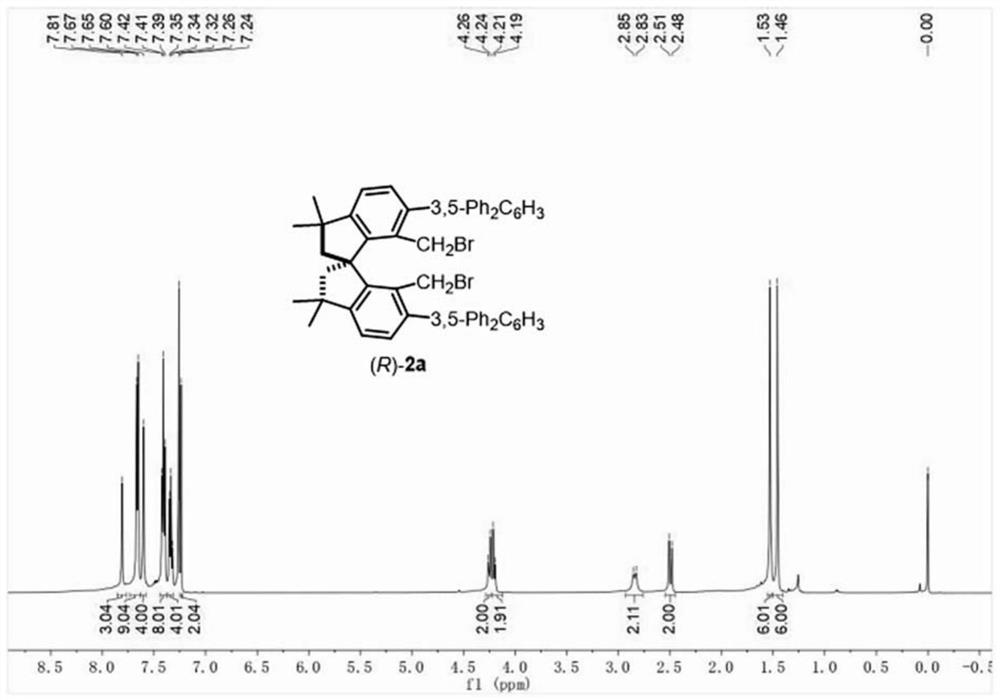
[0060] Under nitrogen protection, (R)-II (1 g, 1.6 mmol), tetrakis(triphenylphosphine) palladium (277 mg, 0.24 mmol), 3,5-bis(tert-butyl) were sequentially added to a dry Schlenk tube Phenylboronic acid (1.5g, 6.4mmol), then tetrahydrofuran (50mL), methanol (2mL) and 2M potassium carbonate solution (5mL) were added; the reaction solution was stirred under reflux for 5 hours. After the reaction was complete, the reaction solution was cooled to room temperature, and the reaction solution was concentrated in vacuo. The obtained crude product was extracted with EtOAc (3×100 mL), and the organic phase was washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, filtered, and reduced pressure. The organic solvent was removed by rotary evaporation, and the obtained crude product was purified by silica gel column chromatography, the eluent was ethyl acetate:petroleum ether=1:100-1:50, and (R)-1b was obtained as 1.13g as a white solid, the yield 99%. 1 H ...
Embodiment 3
[0065] According to the reaction process similar to Example 2, the following chiral compounds can be prepared, and the structure, yield and characterization data are as follows:
[0066]
[0067] According to the reaction process similar to Example 2, the reaction time was 15 hours, the crude product was recrystallized with dichloromethane:petroleum ether=1:8, the yield was 99%, and it was a white solid; 1H NMR (500MHz, CDCl 3 )δ7.43(2H, t, J=1.8Hz),7.41(2H,d,J=8.0Hz),7.35(2H,d,J=7.8Hz),7.18(4H,br),4.81 (2H, d,J=14.0Hz),4.62(2H,d,J=14.0Hz),3.26-3.37(2H,m),2.69-2.77(2H,m), 2.58(2H,d,J=13.0Hz), 2.41-2.56(4H,m),2.37(2H,d,J=13.0Hz),1.59(6H,s), 1.56(6H,s),1.33(36H,s); 13 C NMR (125MHz, CDCl 3 )δ152.58, 152.34, 152.08, 145.78, 139.59, 131.67, 126.24, 124.80, 121.92, 117.81, 61.11, 61.08, 57.45, 57.10, 42.25, 35.21, 32.70, 31.66. M] + calcd for C 55 h 74 NO + 764.5765,found:764.5764.Mp 233-234℃.[α] D 25 =+136.0° (c=1.0, CHCl 3 ).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap