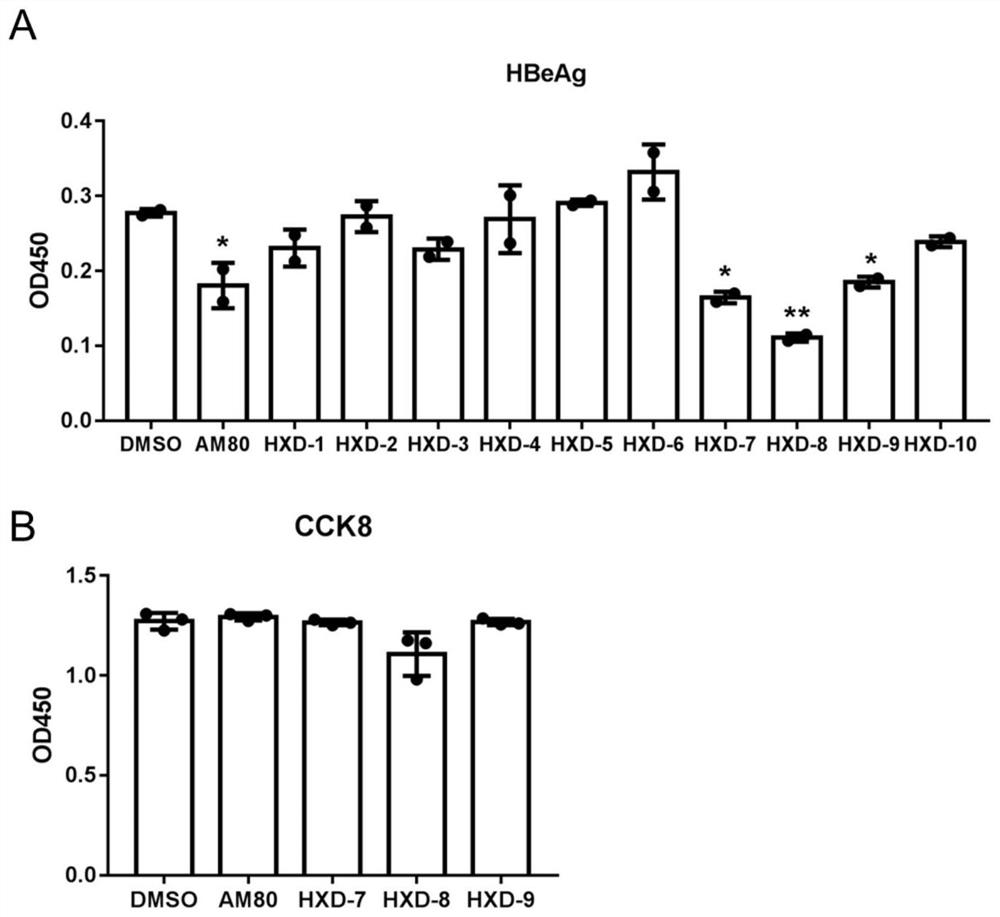
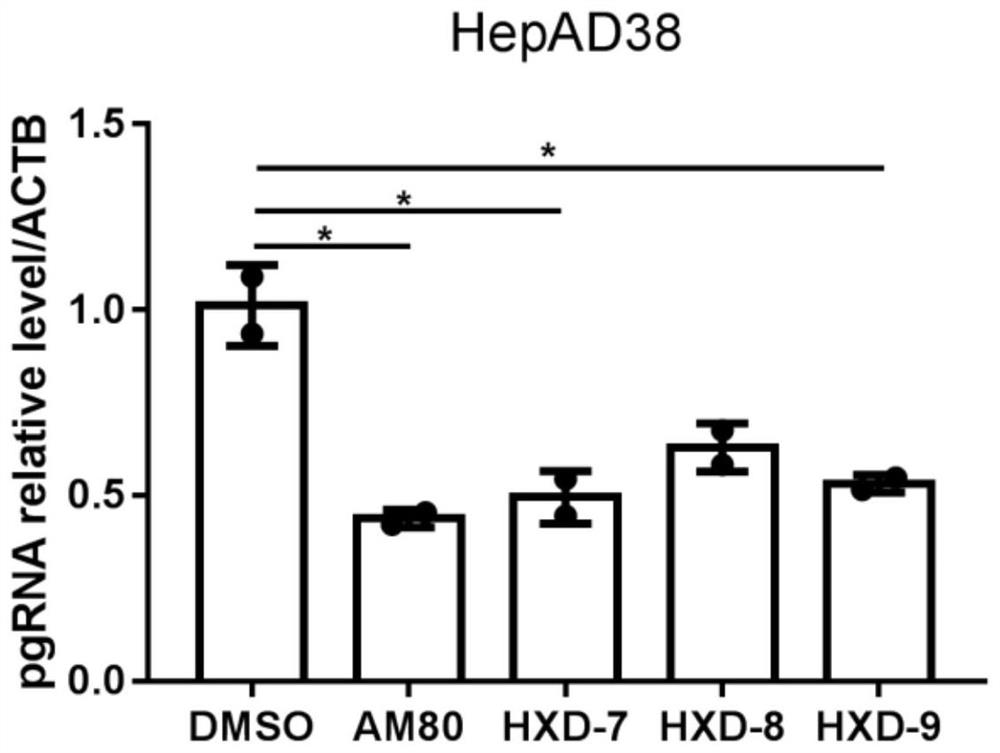
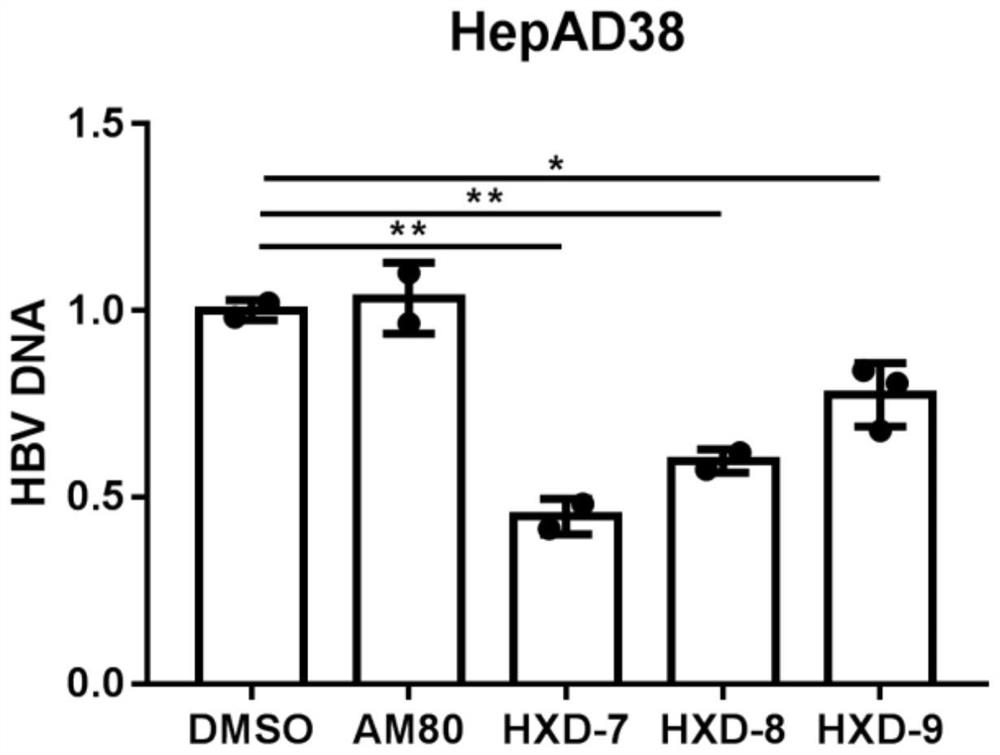
Indole compound with optical activity as well as synthesis method and application thereof
An indole compound and optically active technology, which is applied in the field of optically active indole compounds, can solve the problem of ineffective synthesis of cyano-containing quaternary carbon chiral centers, and achieve good anti-hepatitis B virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Embodiment 1: With N-(2-(5,5-dicyano-6-phenylhexane-1-yne-alkyl)phenyl)-4-methylbenzenesulfonamide as standard substrate, for Study on reaction solvents of palladium-catalyzed synthesis of indoles with cyano-chiral quaternary carbon centers at the β-position:
[0038]
[0039]
[0040] Wherein mol% refers to the relative molar weight, equiv represents the equivalent, the yield is the NMR yield, and the brackets are the separation yield. The amount of ligand in condition 10 is 12mol%, and the amount of ligand in condition 11 is 5mol%. Phase determination, condition 5 or condition 10 is the best reaction condition.
Embodiment 2
[0041] Embodiment 2: With N-(2-(5,5-dicyano-6-phenylhexane-1-yne-alkyl)phenyl)-4-methylbenzenesulfonamide as standard substrate, for Palladium-catalyzed reaction catalyst precursors for the synthesis of indoles containing cyano-chiral quaternary carbon centers at the β-position:
[0042]
[0043]
[0044]
[0045] Among them, mol% refers to the relative molar weight, equiv represents the equivalent, and the yield is the nuclear magnetic yield. Condition 7 adds 20mol% AgSbF 6 , Condition 1 is the best reaction condition, er is determined by high performance liquid chromatography.
Embodiment 3
[0046] Example 3: With N-(2-(5,5-dicyano-6-phenylhexane-1-yne-alkyl)phenyl)-4-methylbenzenesulfonamide as standard substrate, for Palladium-catalyzed reaction ligands for the synthesis of indoles with cyano-containing chiral quaternary carbon centers at the β-position:
[0047]
[0048]
[0049]
[0050]
[0051] Wherein mol% refers to relative molar weight, equiv represents equivalent, yield is NMR yield, er is determined by high performance liquid phase, and condition 2 is the best reaction condition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com