Aromatic heterocyclic amine derivative as well as preparation method and application thereof
A technology of derivatives and heteroamines, applied in the field of compound synthesis, can solve the problems of poor druggability, poor water solubility, and low anti-HBV activity, and achieve good water solubility, large application prospects, and good druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
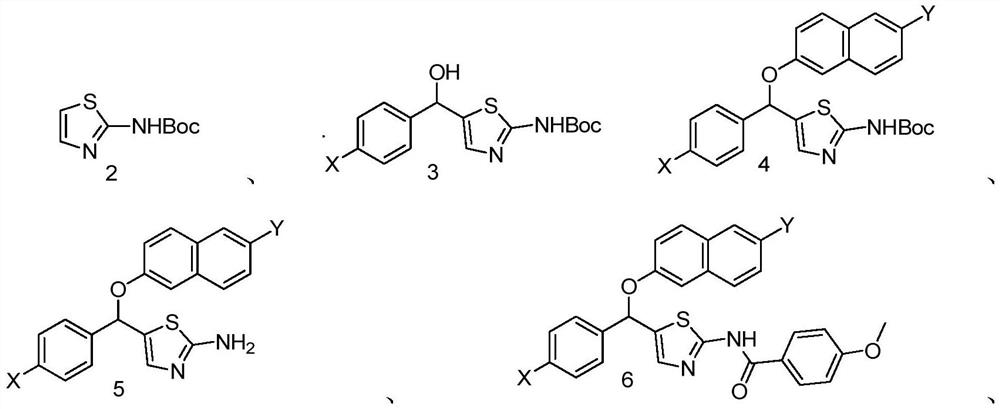
[0032] The preparation method of a new class of aromatic heteroamine derivatives, the basic synthetic route is as follows:
[0033]
[0034] a: Add 2-aminothiazole (compound 1) (2g, 20mmol, 1.0eq) and di-tert-butyl dicarbonate (6.548g, 30mmol, 1.5eq) to a 100mL round-bottomed flask in turn, then add anhydrous tetrahydrofuran to dissolve , react overnight at room temperature. The tetrahydrofuran was spin-dried under reduced pressure and separated by column chromatography to obtain compound 2 (3902 mg, 97.55%).
[0035] b: Add compound 2 (3mmol, 1.0eq) into a 100mL round-bottomed flask, put it on a vacuum pump for 30 minutes, then protect it with argon, add an appropriate amount of anhydrous tetrahydrofuran to dissolve, and put it in a low-temperature reaction bath at -78°C middle. After cooling to -78°C, n-butyllithium (12mmol, 4.0eq) was added slowly over 30 minutes. Then, the corresponding substituted benzaldehyde (4.5mmol, 1.5eq) dissolved in anhydrous THF was slowly a...
Embodiment 1
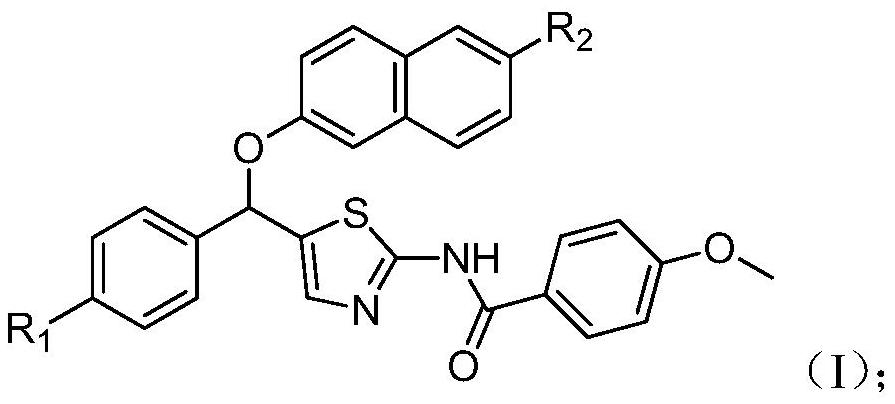
[0043] The chemical structural formula is as the synthesis of aromatic heteroamine derivatives shown in formula (II)
[0044]
[0045] Take the corresponding compound 5 (X is Y is ) (230mg, 0.5mmol), p-methoxybenzoyl chloride (102mg, 0.6mmol), dissolved in anhydrous dichloromethane, then added pyridine (158mg, 2mmol), and reacted overnight at room temperature. The dichloromethane was spin-dried under reduced pressure, and the pH was adjusted to about 4 with 1 mol / L hydrochloric acid, then extracted with ethyl acetate, washed with saturated sodium chloride solution, the organic layer was spin-dried under reduced pressure, and separated by column chromatography to obtain the compound.
[0046] data analysis: 1 H NMR (Bruker 500or 400MHz, the solvent is deuterated methanol, deuterated chloroform or deuterated DMSO), mass spectrometry (ESI, Thermofisher LCQ or QE), the data are as follows:
[0047] 1 H NMR(500MHz,DMSO)δ12.29(s,1H),10.16(s,1H),8.14(s,1H),8.03(d,J=8.9Hz,2H),7...
Embodiment 2
[0050] Synthesis of aromatic heteroamine derivatives with chemical structural formula as shown in formula (III)
[0051]
[0052] Take the corresponding compound 5 (X is Y is ) (376mg, 0.85mmol), p-methoxybenzoyl chloride (145mg, 0.85mmol), dissolved in anhydrous dichloromethane, then added pyridine (135mg, 1.7mmol), and reacted overnight at room temperature. The dichloromethane was spin-dried under reduced pressure, and the pH was adjusted to about 4 with 1 mol / L hydrochloric acid, then extracted with ethyl acetate, washed with saturated sodium chloride solution, the organic layer was spin-dried under reduced pressure, and separated by column chromatography to obtain the compound.
[0053] data analysis: 1 H NMR (Bruker 500or 400MHz, the solvent is deuterated methanol, deuterated chloroform or deuterated DMSO), mass spectrometry (ESI, Thermofisher LCQ or QE), the data are as follows:
[0054] 1 H NMR (500MHz, DMSO) δ12.29(s,1H),10.21(s,1H),8.06(d,J=2.1Hz,1H),8.02(d,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com