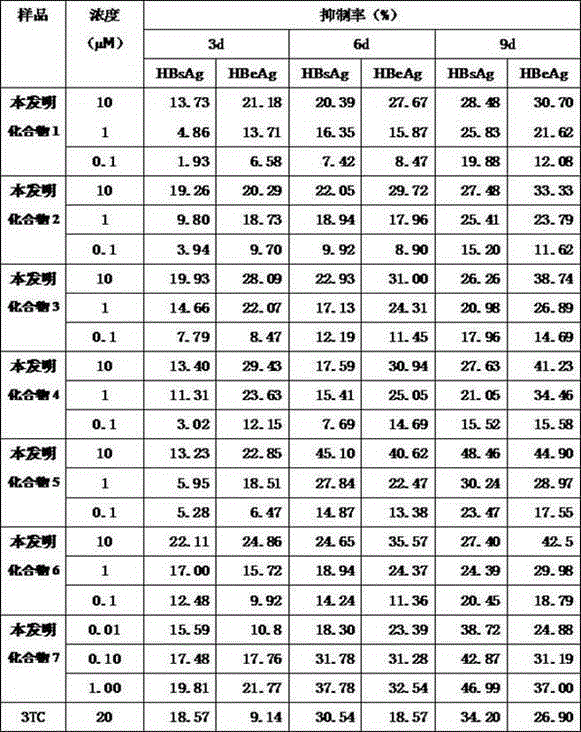
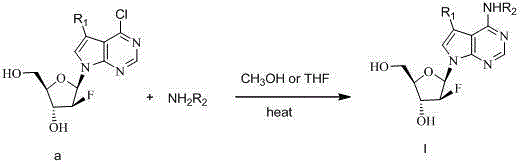
7-deazapurine ribonucleoside compound, synthesis method and pharmaceutical use thereof
A technology of purine ribonucleosides and compounds, which is applied in the field of medicinal chemistry and can solve problems such as the synthesis of 7-deaza-like purine ribonucleoside compounds that have not yet been discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of 4-ethylamino-9-(2′-deoxy-2′-β-fluoro-β-D-furanosyl)pyrrolo[2,3-d]pyrimidine (1)
[0042]In a 50 mL closed reactor, add 4-chloro-9-(2′-deoxy-2′-β-fluoro-3′,5′-di-O-benzoyl-β-D-furanosyl) in sequence Pyrrole[2,3-d]pyrimidine (a) (0.29g, 0.59mmol), 60-70% ethylamine aqueous solution (30mL), tetrahydrofuran (10mL) and methanol (7mL), in 90℃ oil bath, react After 12 hours, TLC detected that the reaction of the raw materials was completed, and after evaporation to dryness, ammonia methanol (30 mL) was added and left overnight. After evaporating to dryness, dichloromethane:methanol=13:1 was used as the eluent and separated by column chromatography to obtain compound (1) (0.17g, 0.59mmol) as white foam, yield: 99.65%. HRMS[M+H]:297.1357; 1 H-NMR(DMSO,400Hz),δ:8.14(1H,s,H-2),7.55-7.53(1H,m,H-NHCH 2 CH 3 ),7.25-7.24(1H,m,H-8),6.63-6.62(1H,m,H-7),6.57-6.52(1H,m,H-1'),5.89-5.88(1H,m, H-O),5.15-5.05(1H,m,H-3'),5.04-5.00(1H,m,H-O),4.37-4.32(1H,m,H-2'),3.79-3.78(1H...
Embodiment 2
[0044] Preparation of 4-(3-aminomethylpyridine)-9-(2′-deoxy-2′-β-fluoro-β-D-furanosyl)pyrrole[2,3-d]pyrimidine (2)
[0045] In a 50 mL closed reactor, add 4-chloro-9-(2′-deoxy-2′-β-fluoro-3′,5′-di-O-benzoyl-β-D-furanosyl) in sequence Pyrrolo[2,3-d]pyrimidine (a) (0.29g, 0.59mmol), 3-aminomethylpyridine (6mL) and tetrahydrofuran (40mL), reacted in an oil bath at 90°C for 12h, detected by TLC, raw material reaction After evaporating to dryness, ammonia methanol (30 mL) was added and left overnight. After evaporating to dryness, use column chromatography to separate and purify, first use dichloromethane:methanol=20:1 as the eluent to remove 3-aminomethylpyridine, and then use 2% triethylamine in dichloromethane:methanol=3: 1 was the eluent, and finally ethyl acetate:methanol=10:1 was used as the eluent to obtain compound (2) (0.14g, 0.28mmol) as a white foam, yield: 47.23%. HRMS[M+H]:360.1472; 1 H-NMR(DMSO,400Hz),δ:8.58(1H,s,H-2),8.45-8.44(1H,m,H-N),8.18-8.17(1H,m,H-8),8.16-8....
Embodiment 3
[0047] Preparation of 4-(3-methoxypropylamino)-9-(2′-deoxy-2′-β-fluoro-β-D-furanosyl)pyrrole[2,3-d]pyrimidine (3)
[0048] In a 50 mL closed reactor, add 4-chloro-9-(2′-deoxy-2′-β-fluoro-3′,5′-di-O-benzoyl-β-D-furanosyl) in sequence Pyrrole[2,3-d]pyrimidine (a) (0.32g, 0.64mmol), 3-methoxypropylamine (6mL) and tetrahydrofuran (40mL), reacted in an oil bath at 90°C for 12h, detected by TLC, raw material reaction After evaporating to dryness, ammonia methanol (30 mL) was added and left overnight. After evaporation to dryness, it was separated and purified by column chromatography, and eluted with dichloromethane:methanol=10:1 as the eluent to obtain compound (3) (0.21g, 0.62mmol) as a white foam, yield: 96.59%. HRMS[M+H]:341.1620; 1 H-NMR(DMSO,400Hz),δ:8.14(1H,s,H-2),7.56(1H,brs,H-N),7.26-7.25(1H,m,H-8),6.64-6.63(1H, m,H-7),6.57-6.56(1H,m,H-1'),5.89-5.88(1H,m,H-O),5.15-5.00(1H,m,H-3'),5.07-5.04( 1H,m,H-O),4.38-4.32(1H,m,H-4'),3.79-3.78(1H,m,H-2'),3.64-3.61(2H,m,H-5'),3.51 -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com