High-temperature-resistant polymer containing m-carborane group and preparation method of high-temperature-resistant polymer
A technology of high-temperature-resistant polymers and dichlorophenyl-m-carborane, which is applied in the field of high-temperature-resistant polymers and ceramic precursors, can solve the problems of difficult synthesis, high toxicity of raw materials, and low yield, and achieve access and use Convenience and safety, low chemical toxicity and stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The present invention also proposes a method for preparing a high-temperature-resistant polymer containing m-carborane groups as described above, comprising the steps of:
[0027] S1: Under anhydrous and oxygen-free conditions, react n-butyllithium and m-carborane in an aprotic solvent to generate carborane lithium salt, then add cuprous salt and the cuprous salt ligand to obtain carborane alkane-copper mixture, and then adding p-chloroiodobenzene or chloriodobenzene to the carborane-copper mixture, heating and reacting to obtain dichlorophenyl-m-carborane monomer; the reaction yield is about 75-90%.
[0028] S2: Under anhydrous and oxygen-free conditions, add the main catalyst, co-catalyst, reducing agent, main catalyst main ligand, main catalyst auxiliary ligand and polar aprotic solvent, heat the reaction, and then add the dichlorophenyl The carborane monomer is refluxed at 55-80°C for 10-24 hours, and finally the reaction is quenched with hydrochloric acid / methanol ...
Embodiment 1
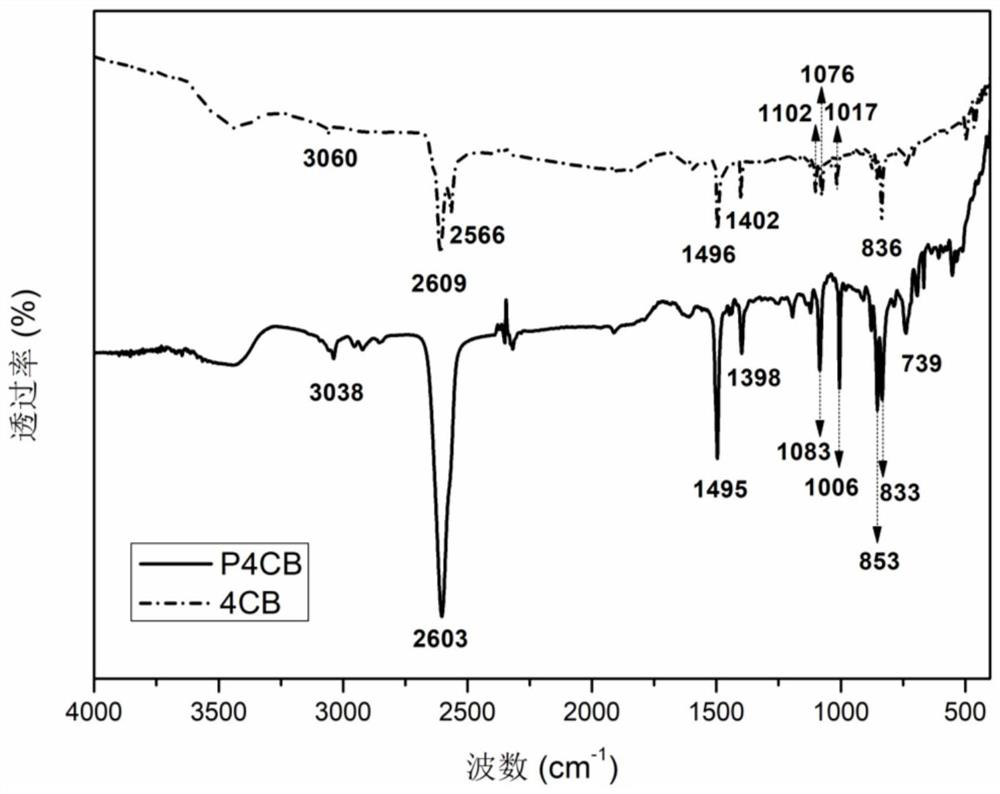
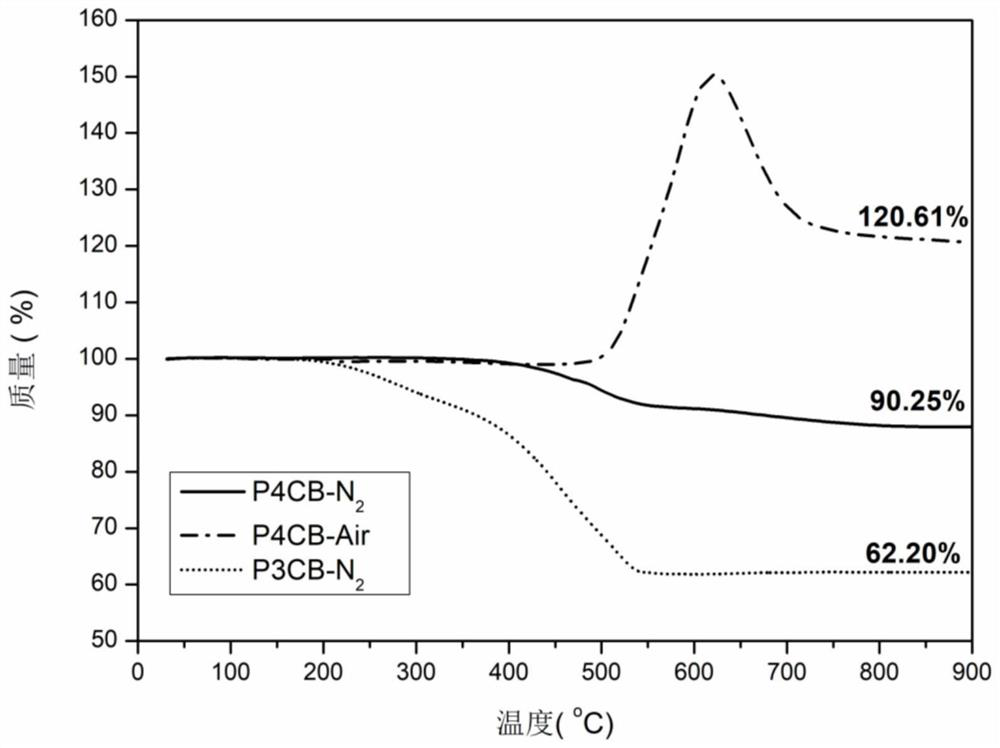
[0059] This embodiment provides a high-temperature-resistant polymer containing m-carborane groups, the high-temperature-resistant polymer is poly[bis(4-chlorophenyl) m-carborane], the chemical structural formula is (1),
[0060]
[0061] Wherein, n is the degree of polymerization, and n takes an integer of 2-300.
[0062] This embodiment also provides a method for preparing a high-temperature-resistant polymer containing m-carborane groups as described above, comprising the following steps:
[0063] The first part, monomer synthesis:
[0064] 1. Add 2.8847g (0.02mol) m-carborane and a stirring magnet into a glass reactor and seal it.
[0065] 2. Repeat the process of evacuating the reaction vessel and filling it with nitrogen three times.
[0066] 3. Add 50 mL of anhydrous ethylene glycol dimethyl ether into the reaction vessel, and m-carborane dissolves after stirring.
[0067] 4. After applying an ice-water bath for several minutes, slowly add 26 mL (0.0416 mol) of 1....
Embodiment 2
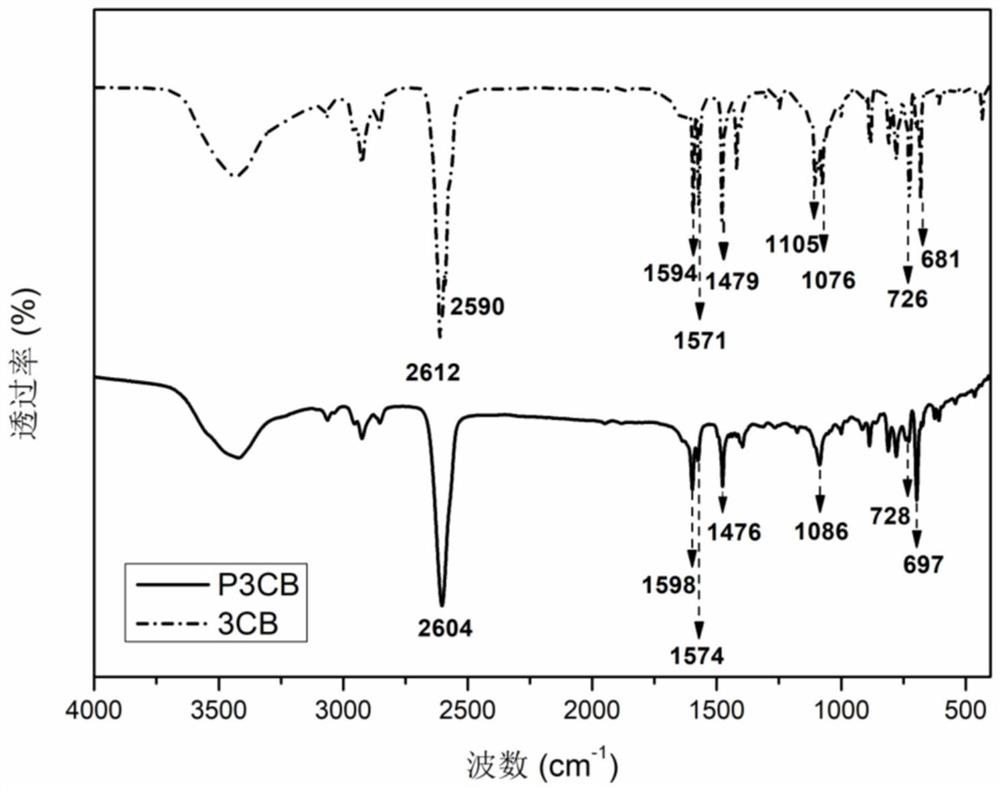
[0086] This embodiment provides a high temperature resistant polymer containing m-carborane group, the high temperature resistant polymer is poly[bis(3-chlorophenyl) m-carborane], the chemical structural formula is (2),
[0087]
[0088] Wherein, n is the degree of polymerization, and n takes an integer of 2-300.
[0089] This embodiment also provides a method for preparing a high-temperature-resistant polymer containing m-carborane groups as described above, comprising the following steps:
[0090] The first part, monomer synthesis:
[0091] 1. Add 2.1635g (0.015mol) of m-carborane and a stirring magnet into a glass reactor and seal it.
[0092] 2. Repeat the process of evacuating the reaction vessel and filling it with nitrogen three times.
[0093] 3. Add 60 mL of anhydrous ethylene glycol dimethyl ether into the reaction vessel, and m-carborane dissolves after stirring.
[0094] 4. After applying an ice-water bath for several minutes, slowly add 19.5 mL (0.0312 mol) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com