Bivalirudin injection preparation and preparation method thereof
A technology for bivalirudin and injection preparations, which is applied in the field of medicine and can solve the problems of long time for bivalirudin to separate out and make liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] ① Measure 80% of the prescription amount (100ml’) of purified water into a beaker, and the water temperature is 30°C;
[0020] ②Add 2.5g of mannitol and magnetically stir until completely dissolved;
[0021] ③Add 5.0g (calculated as bivalirudin) of the crude drug, and magnetically stir until completely dissolved;
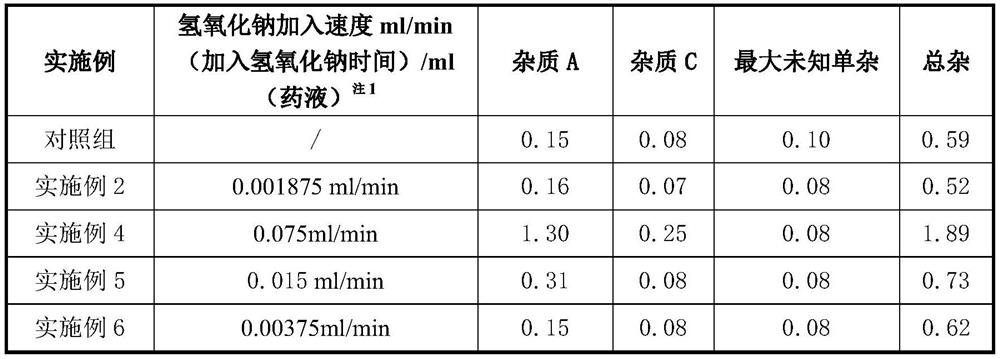
[0022] ④Use 15% (w / v) sodium hydroxide to adjust the pH of the solution, and control the addition rate of sodium hydroxide to 0.001875ml / min (time for adding sodium hydroxide) / ml (medicine solution) by a peristaltic pump 注1 ;
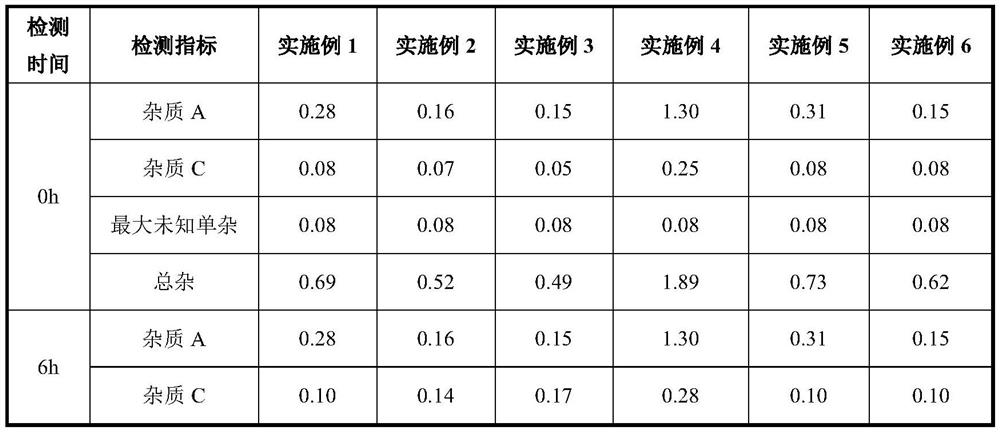
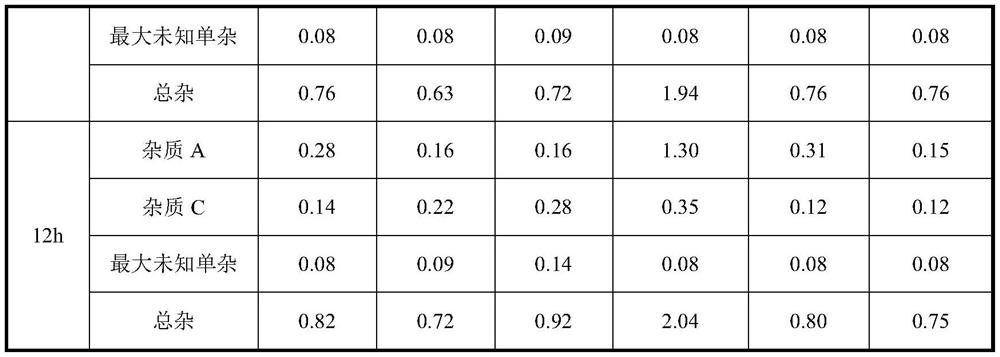
[0023] ⑤ Set the volume by weight (the density is 1.025g / ml), stir for 30 minutes after constant volume, and finally measure the pH of the liquid to 5-6. After constant volume, the drug solution was placed at ≤25°C to measure the stability of the drug solution within 12 hours.
[0024] Note 1: The amount of sodium hydroxide solution that per ml of liquid medicine is exposed to per minute.
Embodiment 2
[0026] ① Measure 80% of the prescription amount (100ml’) of purified water into a beaker, and the water temperature is 30°C;
[0027] ②Add 2.5g of mannitol and magnetically stir until completely dissolved;
[0028] ③Add 5.0g (calculated as bivalirudin) of the crude drug, and magnetically stir until completely dissolved;
[0029] 4. Regulate the pH of the solution with 5% (w / v) sodium hydroxide, and control the sodium hydroxide addition rate by a peristaltic pump to be 0.001875ml / min (the time of adding sodium hydroxide) / ml (medicine solution);
[0030] ⑤ Set the volume by weight (the density is 1.025g / ml), stir for 30 minutes after constant volume, and finally measure the pH of the liquid to 5-6. After constant volume, the drug solution was placed at ≤25°C to measure the stability of the drug solution within 12 hours.
Embodiment 3
[0032] ① Measure 80% of the prescription amount (100ml’) of purified water into a beaker, and the water temperature is 30°C;
[0033] ②Add 2.5g of mannitol and magnetically stir until completely dissolved;
[0034] ③Add 5.0g (calculated as bivalirudin) of the crude drug, and magnetically stir until completely dissolved;
[0035] 4. Regulate the pH of the solution with 5% (w / v) sodium hydroxide, and control the sodium hydroxide addition rate by a peristaltic pump to be 0.001875ml / min (the time of adding sodium hydroxide) / ml (medicine solution);
[0036] ⑤ Set the volume by weight (the density is 1.025g / ml), stir for 30 minutes after constant volume, and then measure the pH of the liquid.
[0037] After constant volume, the medicinal solution was placed at 30°C to measure the stability of the medicinal solution within 12 hours.
[0038] Conclusion: Through the comparison of Example 2 and Example 3, with the prolongation of time, the impurity C has a tendency to increase, but a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com