Fluorodinitroethoxy furazan pyrazine compounds and synthesis method thereof
A technology of fluorodinitroethoxyfurur and dinitroethoxy, which is applied in the field of energetic material synthesis, can solve the problems of high solvent toxicity and insufficient mild reaction conditions, and achieve simple steps, mild synthesis conditions, and extensive The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
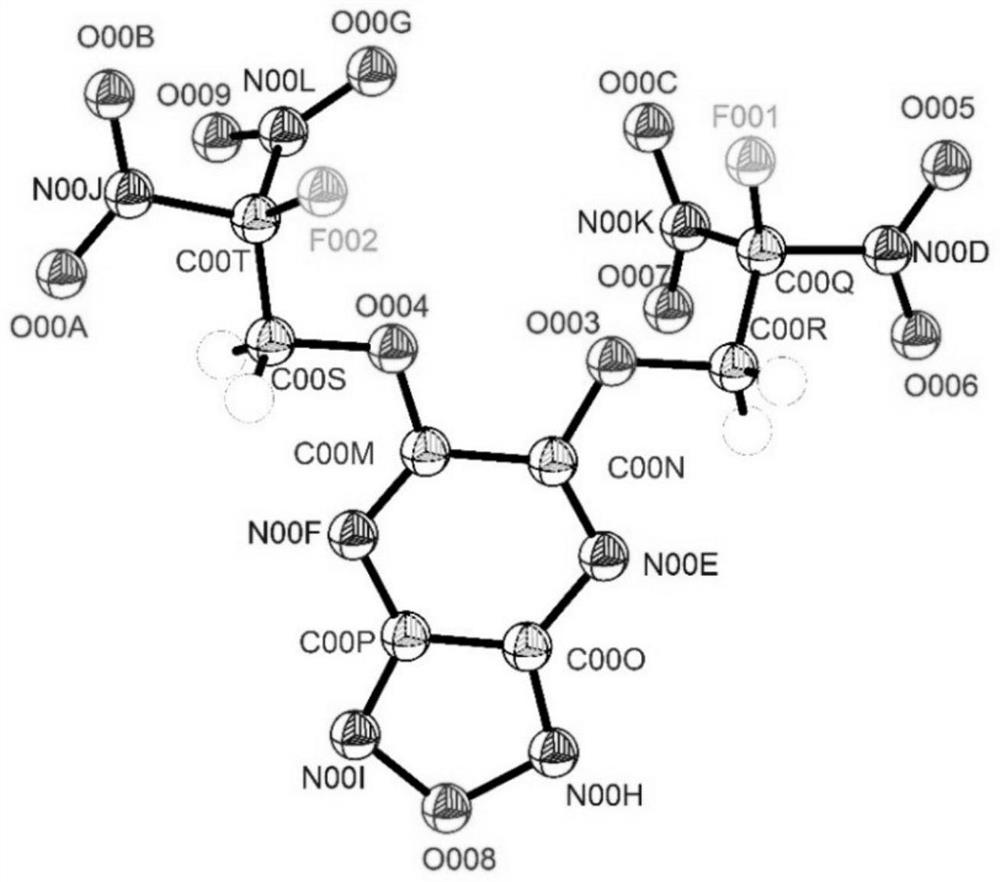
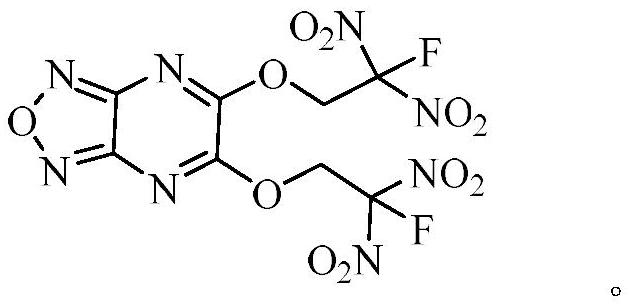
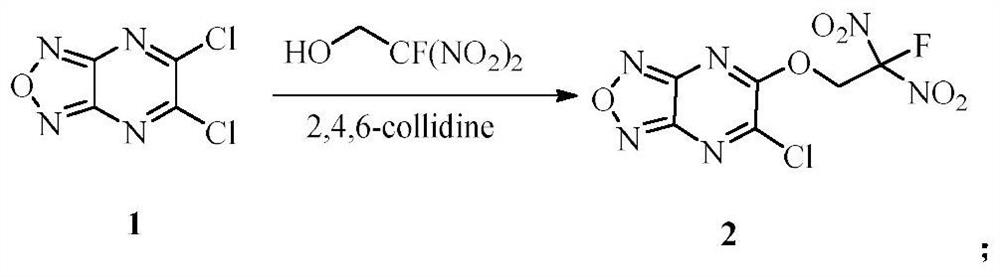
[0028] The preparation method of the target product of the present invention comprises the following steps: (1) dissolving 5,6-dichlorofurazo[3,4-b]pyrazine (1) in dichloromethane, adding 2-fluoro-2,2-dinitroethanol, 2,4,6-collidine, then stirred, filtered through a short column of silica gel, eluted with dichloromethane and the eluent was concentrated under reduced pressure , to obtain 5-chloro-6-(2-fluoro-2,2-dinitroethoxy)furazo[3,4-b]pyrazine (2); (2) compound 5-chloro-6 -(2-Fluoro-2,2-dinitroethoxy)furazo[3,4-b]pyrazine (2) was dissolved in dichloromethane, and 2-fluoro-2,2 -Dinitroethanol, 4-dimethylaminopyridine, stirred, filtered through a short column of silica gel, eluted with dichloromethane and concentrated under reduced pressure to obtain 5,6-bis(2-fluoro-2, 2-Dinitroethoxy)furazano[3,4-b]pyrazine (3).
[0029]
Embodiment 1
[0031] Synthesis of 5,6-bis(2-fluoro-2,2-dinitroethoxy)furazo[3,4-b]pyrazine:
[0032] (1) Dissolve 5,6-dichlorofurazo[3,4-b]pyrazine (1) (152mg, 0.8mmol) in dichloromethane (6mL), and add 2-fluoro-2 , 2-dinitroethanol (126mg, 0.8mmol), 2,4,6-collidine (99mg, 0.8mmol), then stirred it at room temperature (25°C) for 1h, filtered through a short column of silica gel, Elute with dichloromethane and concentrate the eluent under reduced pressure to obtain 5-chloro-6-(2-fluoro-2,2-dinitroethoxy)furazo[3,4-b]pyridine azine (2). (2) Compound 2 (200mg, 0.65mmol) was dissolved in dichloromethane (4mL), and 2-fluoro-2,2-dinitroethanol (100mg, 0.65mmol), 4-dimethyl Aminopyridine (80 mg, 0.65 mmol). The reaction was stirred at room temperature (25°C) for 1 h, filtered through a short column of silica gel, eluted with dichloromethane and the eluent was concentrated under reduced pressure to obtain 5,6-bis(2-fluoro-2,2-bis Nitroethoxy)furazo[3,4-b]pyrazine (3) (240 mg, 87% yield). Its c...
Embodiment 2
[0037] The reaction temperature of step (1) and step (2) is 40 DEG C, and other conditions are the same as Example 1, and the yield is 72%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap