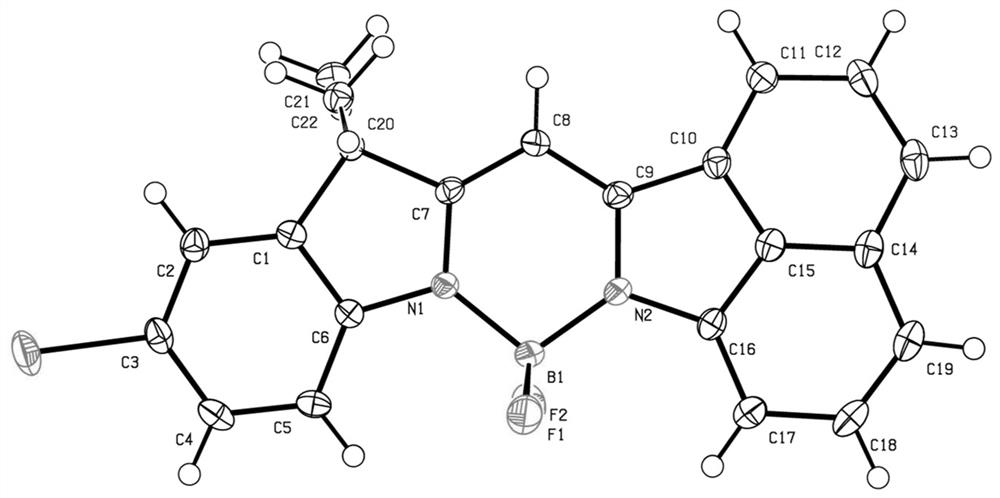
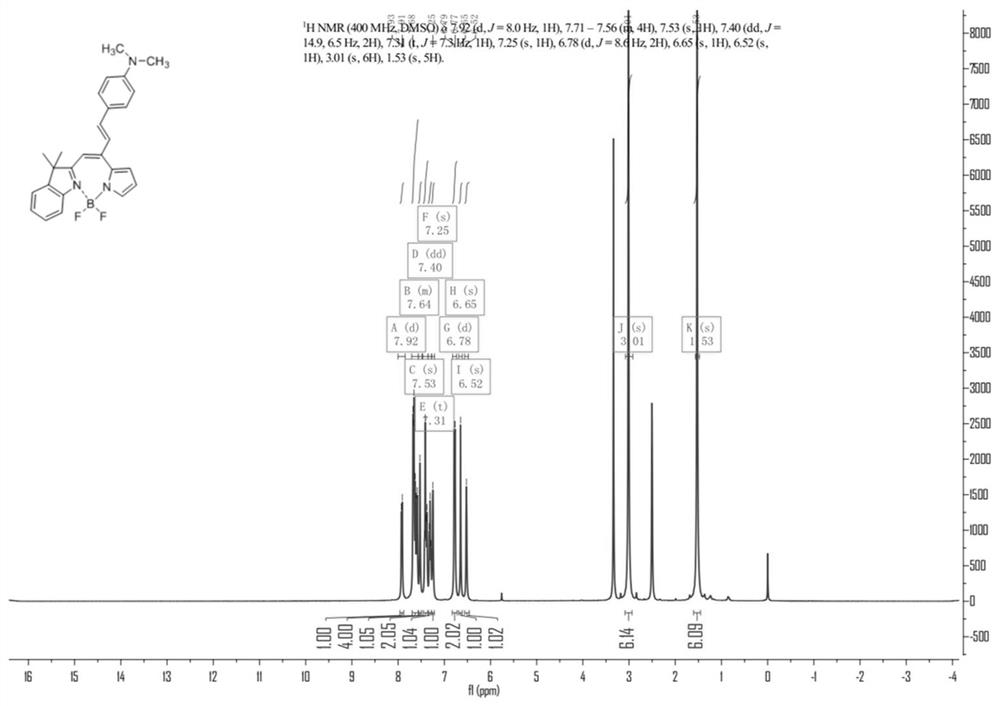
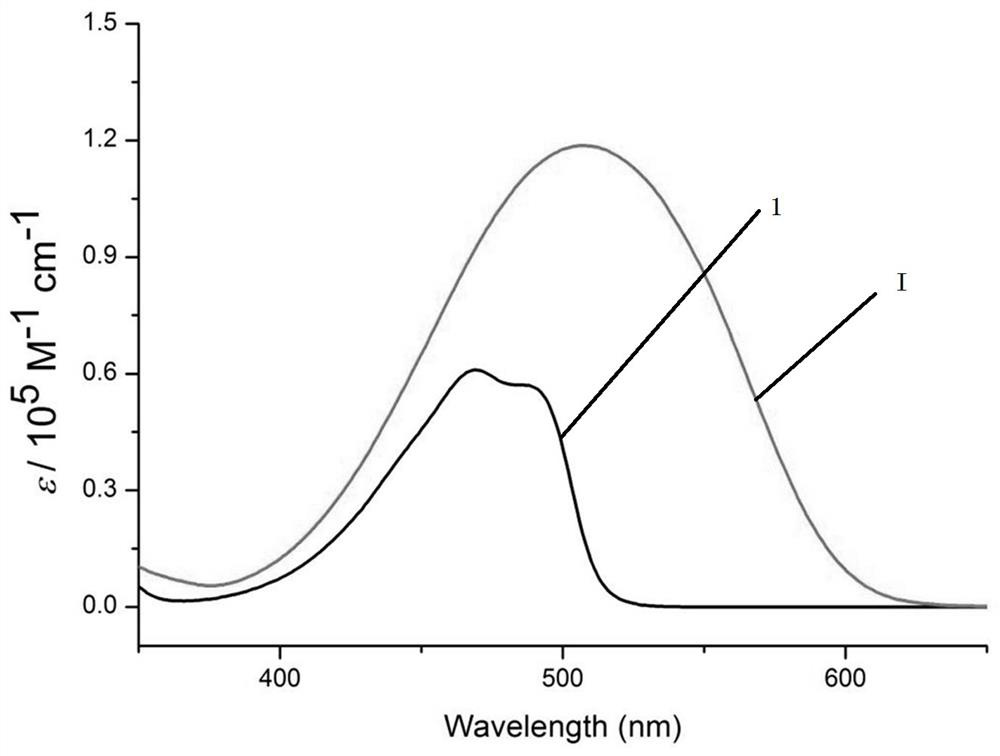
A kind of preparation method of bopyin fluorescent dye responsive to trifluoroacetic acid
A fluorescent dye and trifluoroacetic acid technology, applied in the field of BOPYIN fluorescent dye preparation, can solve the problems of long reaction time, low sensitivity, and long time consumption, and achieve the effects of easy control of reaction conditions, simple product purification, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Compound 1 (298 mg, 1 mmol) was weighed, 30.00 mL of toluene was mixed and dissolved, p-dimethylaminobenzaldehyde (149 mg, 1 mmol), piperidine (0.09 ml, 1 mmol), acetic acid (0.06 ml, 1 mmol) were added in sequence, The reaction was completed by heating and stirring at 100° C. for 12 hours, the reactant was rotary evaporated, and purified by column chromatography to obtain a dark green solid I (134.6 mg) with a yield of 31.3%.
[0028]
Embodiment 2
[0030] Compound 1 (298mg, 1mmol) was weighed, 30.00mL of toluene was mixed and dissolved, and p-dimethylaminobenzaldehyde (298mg, 2mmol), piperidine (0.09ml, 1mmol), acetic acid (0.06ml, 1mmol) were added in sequence, The reaction was completed by heating and stirring at 100° C. for 10 hours, the reactant was rotary evaporated, and purified by column chromatography to obtain a dark green solid I (157.4 mg) with a yield of 36.6%. When the amount of p-dimethylaminobenzaldehyde was increased by a factor of 2, the yield was increased by 5.3%, and the reaction time was shortened by 2 hours.
Embodiment 3
[0032] Compound 1 (298mg, 1mmol) was weighed, 30.00mL of toluene was mixed and dissolved, p-dimethylaminobenzaldehyde (298mg, 2mmol), piperidine (0.18ml, 2mmol), acetic acid (0.12ml, 2mmol) were added successively, The reaction was completed by heating and stirring at 100° C. for 10 hours, the reactant was rotary evaporated, and purified by column chromatography to obtain a dark green solid I (160.4 mg) with a yield of 37.3%. When the amount of piperidine, acetic acid was increased by a factor of 2 corresponding to Example 2, the yield did not change significantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com