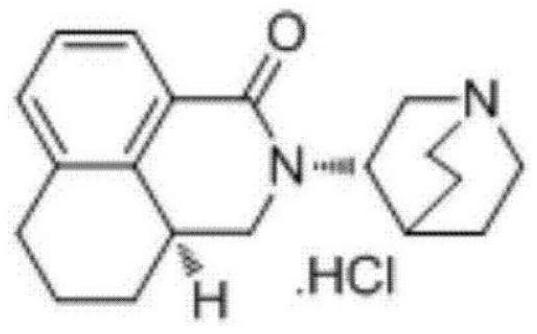
Preparation method of high-purity palonosetron hydrochloride
A palonosetron, high-purity technology, applied in the field of preparation of high-purity palonosetron hydrochloride, can solve the problems of low yield, unstable process, low purity of palonosetron hydrochloride, etc., and achieve saving The effect of cost, environmental protection, and convenience for solvent recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
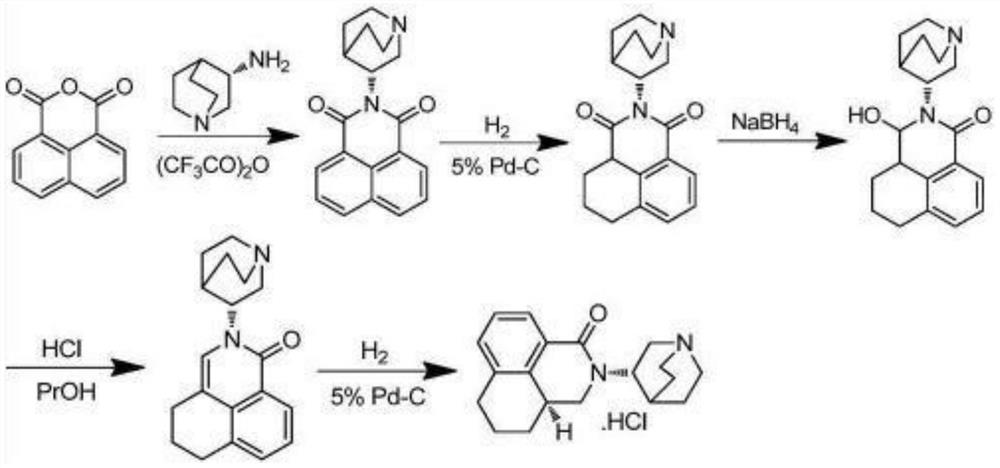
[0048] The invention provides a kind of preparation method of high-purity palonosetron hydrochloride, comprising the following steps:
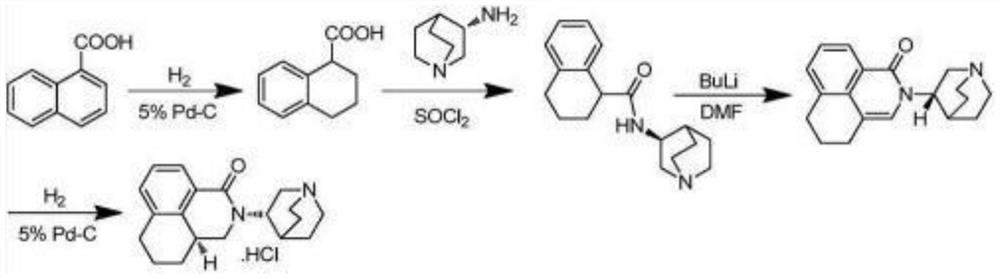
[0049] Step 1), the synthesis of (S)-1,2,3,4-tetrahydronaphthoic acid:
[0050] Add 1,2,3,4-tetrahydronaphthoic acid and quinine into 50% ethanol, stir at 30-60°C to dissolve the solution, TLC detects that the reaction is complete, lower the temperature to below 0°C and place overnight, crystallize, pump Filter, dry, then add ethanol to refine, crystallize below 0°C, filter with suction, dry, add hydrochloric acid and ethyl acetate, separate liquid, dry, filter, concentrate, add n-hexane to the residue for recrystallization to obtain (S) -1,2,3,4-tetrahydronaphthoic acid;
[0051] Step 2), the synthesis of (R)-N-((S)-3-quinyl)-1,2,3,4-tetrahydronaphthyl-1-carboxamide
[0052] Dissolve compound (S)-1,2,3,4-tetrahydronaphthoic acid in ethyl acetate, add oxalyl chloride under stirring, stir at room temperature, add DMF and stir at room temperat...
Embodiment 1
[0116] Step 1), the synthesis of (S)-1,2,3,4-tetrahydronaphthoic acid
[0117] Add 17.6g of 1,2,3,4-tetrahydronaphthoic acid and 32.4g of quinine into 170mL of 50V / V% ethanol, stir at 50°C for 30min to dissolve the solution, TLC detects that the reaction is complete, PE:EA= 1:2, cool down to below 0°C and place overnight, crystallize, filter with suction, dry, then add 100mL ethanol to refine, crystallize below 0°C, filter with suction, dry, add 60mL of 1mol / L hydrochloric acid and 120mL of ethyl acetate The ester was separated, dried, filtered, concentrated, and the residue was recrystallized by adding n-hexane to obtain 7.50 g of a white solid with a yield of 42.6%. The HPLC showed that the purity was 99.81%. , toluene).
[0118] Step 2), the synthesis of (R)-N-((S)-3-quinyl)-1,2,3,4-tetrahydronaphthyl-1-carboxamide
[0119] Dissolve 17.6g (S)-1,2,3,4-tetrahydronaphthoic acid in 50mL ethyl acetate, add 10mL oxalyl chloride under stirring, stir at room temperature for 30min...
Embodiment 2
[0128] Step 1), the synthesis of (S)-1,2,3,4-tetrahydronaphthoic acid
[0129] Add 15.6g of 1,2,3,4-tetrahydronaphthoic acid and 29.8g of quinine into 158mL of 50V / V% ethanol, stir at 50°C for 30min to dissolve the solution, TLC detects that the reaction is complete, PE:EA= 1:2, cool down to below 0°C and place overnight, crystallize, filter with suction, dry, then add 90mL of ethanol to refine, crystallize below 0°C, filter with suction, dry, add 55mL of 1mol / L hydrochloric acid and 100mL of ethyl acetate The ester was separated, dried, filtered, concentrated, and the residue was recrystallized by adding n-hexane to obtain (S)-1,2,3,4-tetrahydronaphthoic acid as a white solid 7.21g, the yield was 43.6%, and the purity was shown by HPLC It was 99.82%, [α]D=-61.0° (c=1.0333, toluene).
[0130] Step 2), the synthesis of (R)-N-((S)-3-quinyl)-1,2,3,4-tetrahydronaphthyl-1-carboxamide
[0131] Dissolve 15.6g (S)-1,2,3,4-tetrahydronaphthoic acid in 50mL ethyl acetate, add 8mL oxaly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com