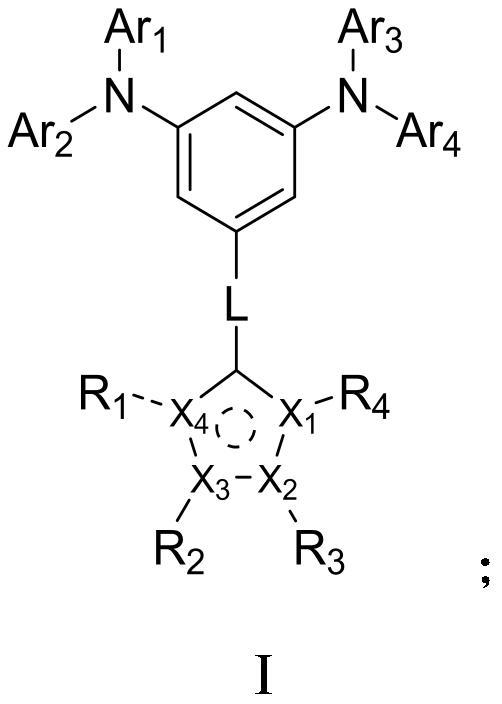
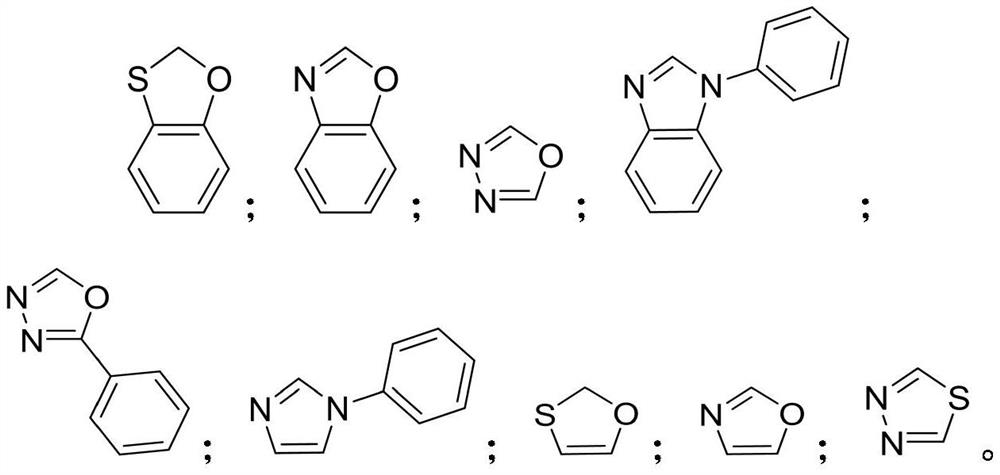
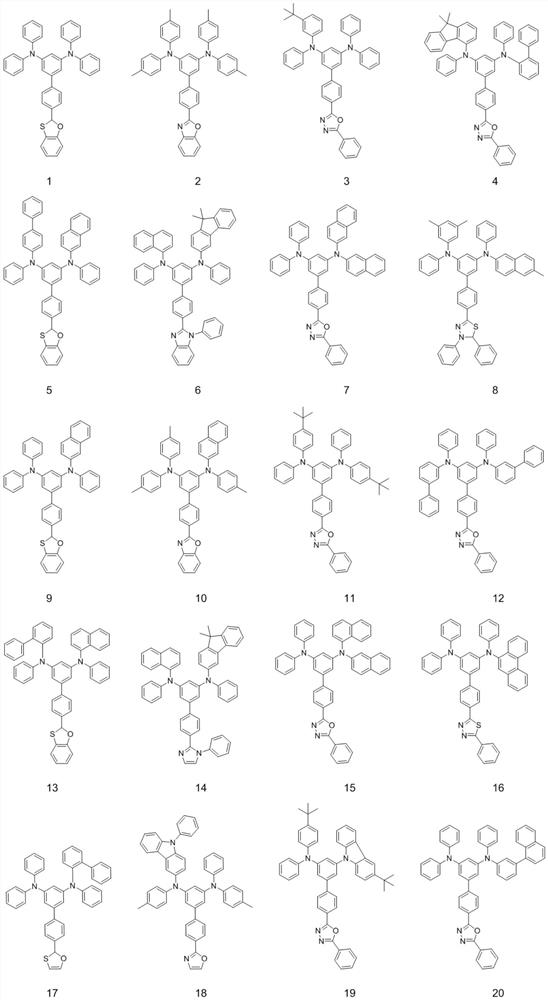
Aromatic amine compound and preparation method, organic electroluminescent device and display device
A technology for display devices and compounds, which is applied in the field of organic electroluminescent devices and display devices, aromatic amine compounds and their preparation, can solve problems such as insufficiently high refractive index, poor light extraction effect, and easy to affect the light emission of light-emitting layer materials, and achieve Increased refractive index, high glass transition temperature, and improved light extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] This embodiment provides an aromatic amine compound, the method of preparation thereof is as follows:
[0065]
[0066] Step 1:
[0067] After adding the chemical formula reactant A-1 (50 mmol) and the reactant B-1 (40 mmol) to the reaction vessel in the toluene, Pd was added under a nitrogen atmosphere 2 (dba) 3 (0.5mmol)、P(t-Bu) 3(2.5mmol)、t-BuONa110mmol)。 After addition, the reaction temperature was slowly warmed to 110 ° C, and the mixture was stirred for 10 h. Using diatomaceous earth while hot filtration, remove salts and catalysts, the filtrate is cooled to room temperature, then distilled water is added to the filtrate for washing, the organic phase is retained after separation, and the aqueous phase is extracted with ethyl acetate. The combined organic layer is then dried using magnesium sulfate and the solvent is removed using a rotary evaporator. Then, with a volume ratio of 1:5 dichloromethane: petroleum ether as the eluent, the remaining substances were purif...
Embodiment 2
[0071] This embodiment provides an aromatic amine compound, the method of preparation thereof is as follows:
[0072]
[0073] Step 1:
[0074] After adding the reactants of the chemical formula A-20 (50 mmol) and the reactants B-20 (40 mmol) in the reaction vessel after dissolving in toluene, Pd was added under a nitrogen atmosphere 2 (dba) 3 (0.5mmol)、P(t-Bu) 3(2.5mmol)、t-BuONa110mmol)。 After addition, the reaction temperature was slowly warmed to 110 ° C, and the mixture was stirred for 10 h. Using diatomaceous earth while hot filtration, remove salts and catalysts, the filtrate is cooled to room temperature, then distilled water is added to the filtrate for washing, the organic phase is retained after separation, and the aqueous phase is extracted with ethyl acetate. The combined organic layer is then dried using magnesium sulfate and the solvent is removed using a rotary evaporator. Then, with a volume ratio of 1:5 dichloromethane: petroleum ether as the eluent, the remaini...
Embodiment 3
[0078] This embodiment provides an aromatic amine compound, the method of preparation thereof is as follows:
[0079]
[0080] Step 1:
[0081] After the reaction formula A-40 (50 mmol) and the reactant B-40 (40 mmol) were added to the reaction vessel after being dissolved in toluene, Pd was added under a nitrogen atmosphere 2 (dba) 3 (0.5mmol)、P(t-Bu) 3(2.5mmol)、t-BuONa110mmol)。 After addition, the reaction temperature was slowly warmed to 110 ° C, and the mixture was stirred for 10 h. Using diatomaceous earth while hot filtration, remove salts and catalysts, the filtrate is cooled to room temperature, then distilled water is added to the filtrate for washing, the organic phase is retained after separation, and the aqueous phase is extracted with ethyl acetate. The combined organic layer is then dried using magnesium sulfate and the solvent is removed using a rotary evaporator. Then, with a volume ratio of 1:5 dichloromethane: petroleum ether as the eluent, the remaining substa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com