Functionalized rubbers
A functionalized, rubbery polymer technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of unstable rubber over time, difficult removal of copper catalysts, low reaction temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
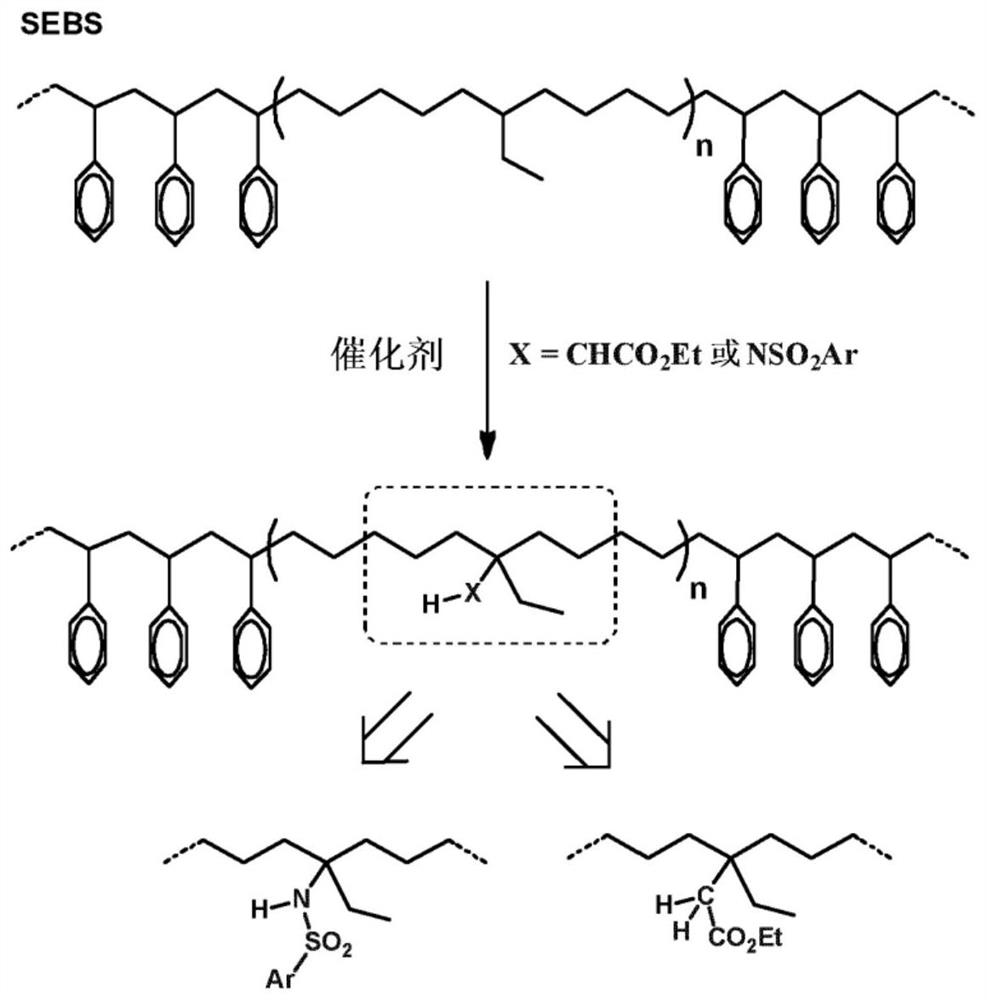
[0142] Example 1. Functionalization of SEBS with [N-(p-toluenesulfonyl)imino]phenyl iodane
[0143] This functionalization reaction was repeated 3 times with different amounts of reactants, see table below for more details.
[0144]The SEBS rubber polymer was dissolved in 1,2-dichloroethane, to which N-(p-toluenesulfonyl)imino]phenyliodinanes (PhINTs) and hydrogen tris(3,5-dimethyl- 4-bromopyrazolyl) borate silver catalyst (Tp* ,Br Ag).
[0145] The mixture was heated at 80° C. for 12 hours under nitrogen atmosphere. After cooling, methanol (100 mL to 200 mL) was added and the precipitated functionalized rubber was isolated by filtration and dried under vacuum. The isolated yield of the polymer was higher than 95%, the incorporation of NHTs units ranged from 1% w / w to 5% w / w (see table below), and was derived from the insertion of NHTs mainly into the tertiary sites of the rubber chain. The side -NHTs groups in the unit correspond.
[0146]
Embodiment 2
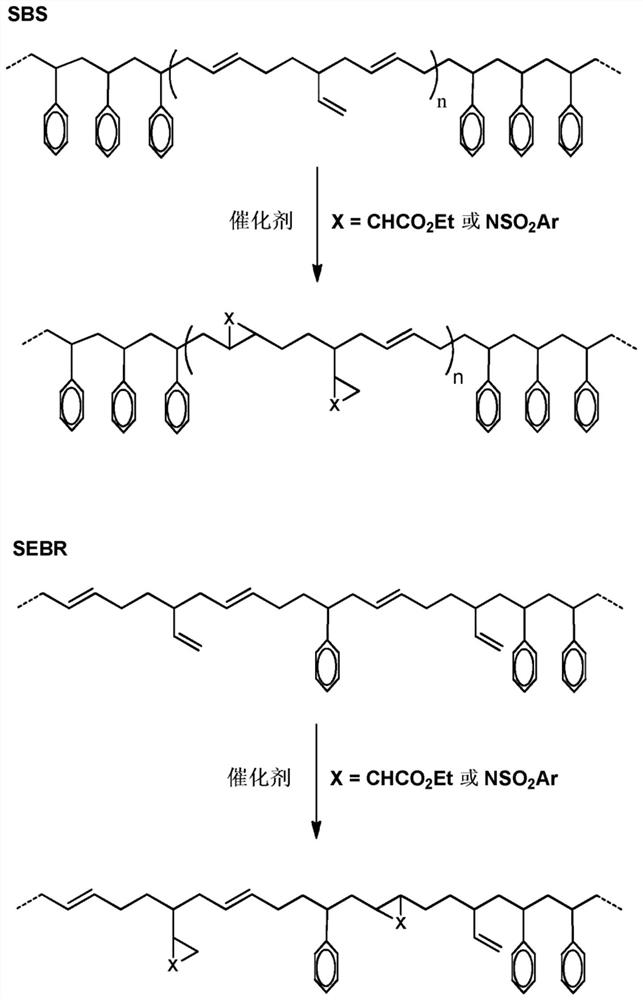
[0147] Example 2. Functionalization of unsaturated rubber with [N-(p-toluenesulfonyl)imino]phenyl iodane
[0148] In a variant of Example 1, and carried out at room temperature in cyclohexane as solvent, modified rubbers are obtained with a degree of incorporation of NHTs units in the range of 1% to 5% in >95% yield. In this case, the unsaturated rubber is modified upon formation of aziridine rings derived from the addition of NHTs units to the double bond of the unsaturated rubber.
Embodiment 3
[0149] Example 3. Functionalization of SEBS with ethyl diazoacetate
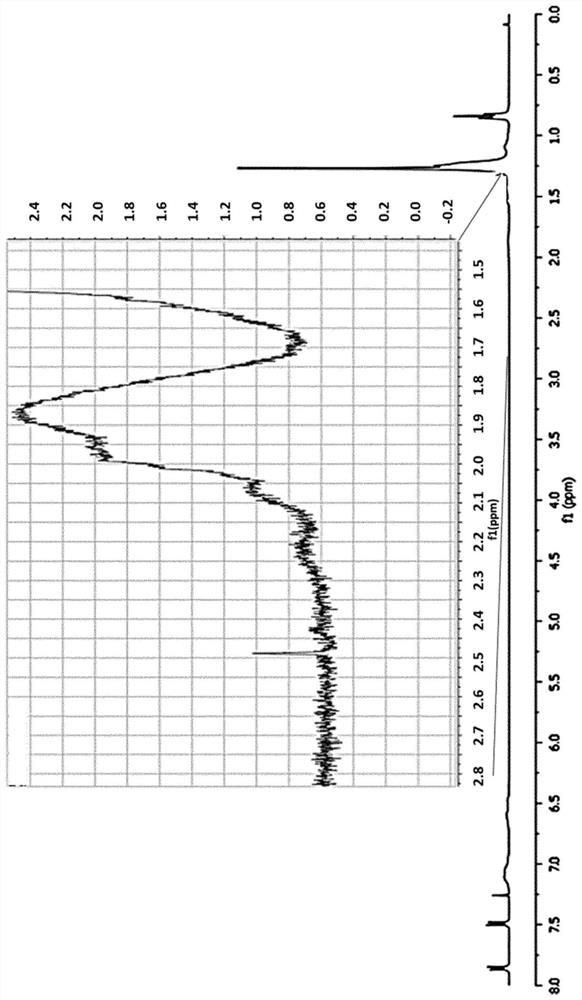
[0150] Cyclohexane solutions of rubber and catalyst at concentrations similar to those described in Example 1 were prepared. Ethyl diazoacetate (EDA) was dissolved in cyclohexane, and the solution was slowly added to the above mixture by means of an automatic addition system at room temperature for 12 hours. The mixture was then stirred for an additional hour before the solvent was removed under reduced pressure, redissolved in a minimum of THF and precipitated with methanol. After filtration and drying under vacuum, >95% of the mass was recovered. 1 H NMR studies show that CHCO 2 1% w / w to 5% w / w of the Et group is incorporated into the polymer chain, which now has a pendant -CH 2 CO 2 Et unit.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com