N glycosyl transferase mutant F13 and application thereof
A technology of glycosyltransferase and mutant, applied in the directions of transferase, enzyme, hydrolase, etc., can solve the problems of incapable of glycosylation of polypeptides, unreported N-glycosyltransferase, complicated steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
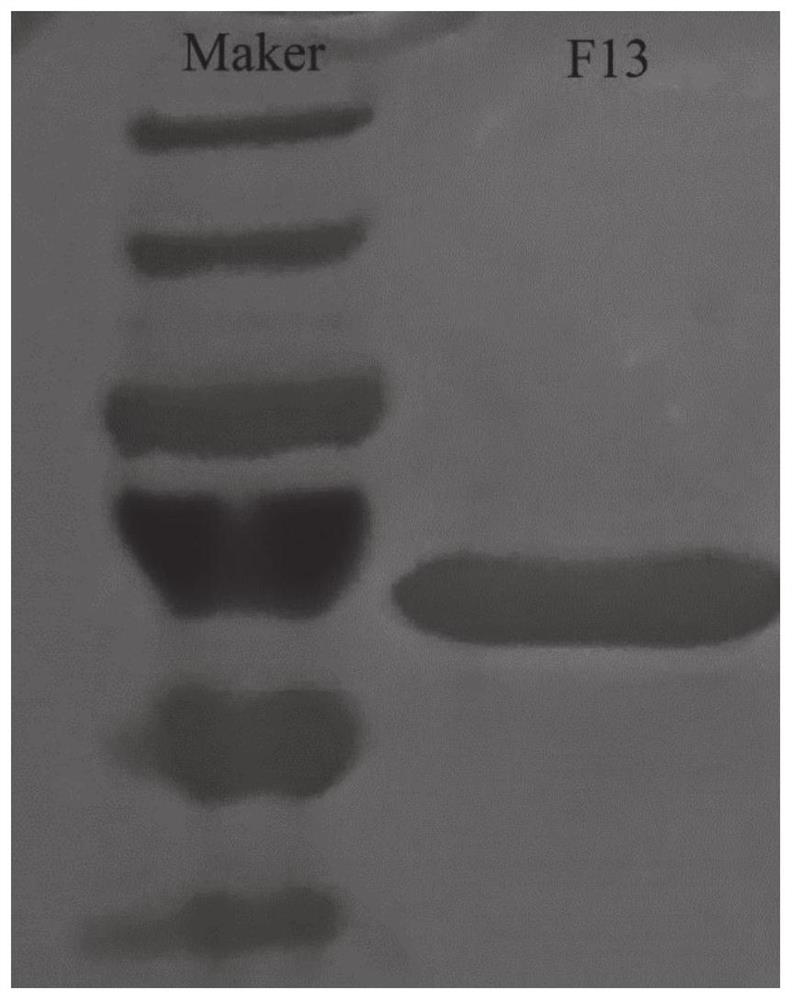
[0028] Example 1: Construction of N-glycosyltransferase ApNGT mutant F13 and expression, purification and identification of F13 protein
[0029] 1. Construction of expression strains
[0030] In the early stage, a large number of NGT site-directed mutation strains were constructed and mutant F13 was screened. The N-glycosyltransferase gene in wild-type N-glycosyltransferase (NCBI Reference Sequence: WP_005605627.1) Actinobacillus pleuropneumoniae was synthesized by Nanjing GenScript and constructed on the vector pET45b, which is commercialized The strain is DH5α-pET45b-ApNGT strain.
[0031] Use the DH5α-pET45b-ApNGT strain, culture the DH5α-pET45b-ApNGT strain in advance with 0.1 mg / mL LB liquid medium at 170 rpm at 37°C to OD 600 Reach 0.6, and mention the plasmid as a template.
[0032] According to Novizym one-step multi-point mutagenesis kit (C25-01) instructions, combined with Novizym online primer design program CE Design to design mutagenic primers (see Table 2). N...
Embodiment 2
[0040] Example 2: Application of N-glycosyltransferase mutant F13 in polypeptide glycosylation modification
[0041] Using UDP-Glc / UDP-Gal / UDP-GlcA as sugar donors, react with 20uL of the reaction system shown in Table 3 at 37°C for 3h, heat at 100°C for 10min after the reaction, centrifuge at 12000rpm for 10min, take the supernatant and dilute 100 times, used for ESI-MS detection. Among them, the substrate peptides used for experimental verification are shown in Table 4.
[0042] Table 3: Reaction system.
[0043]
[0044] Table 4: Substrate peptides used for experimental validation
[0045]
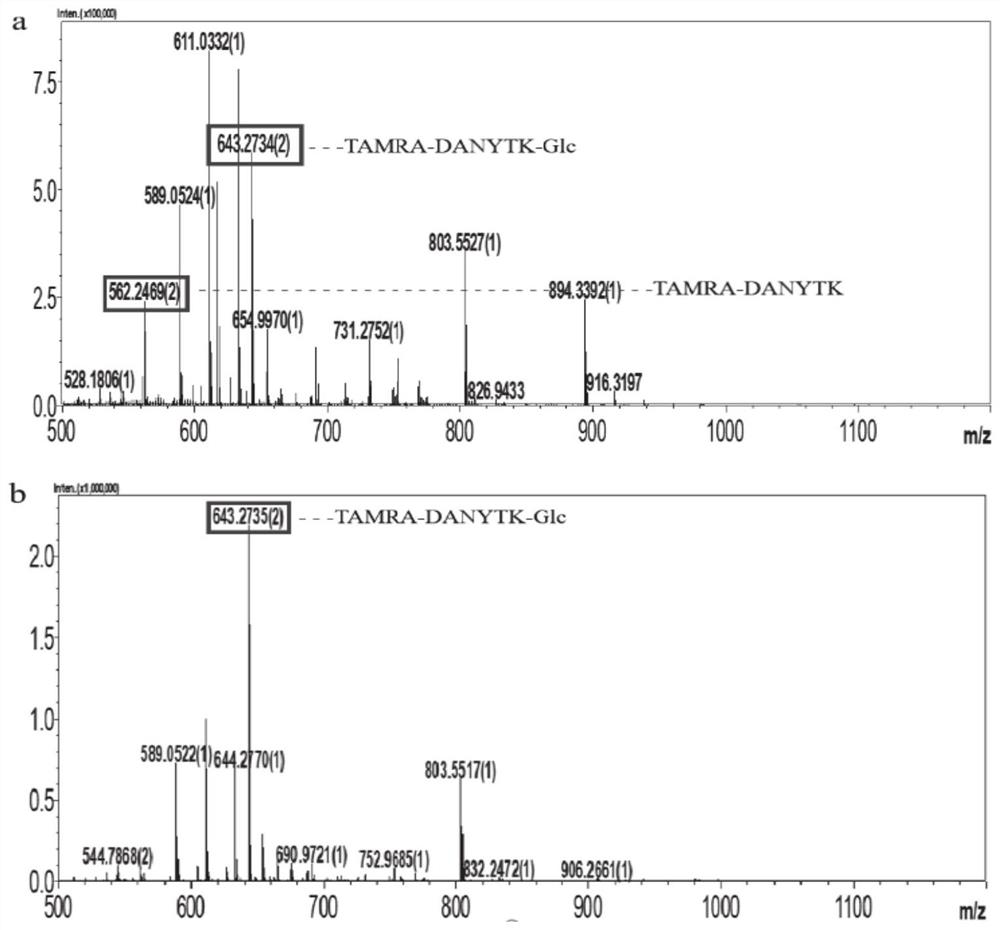
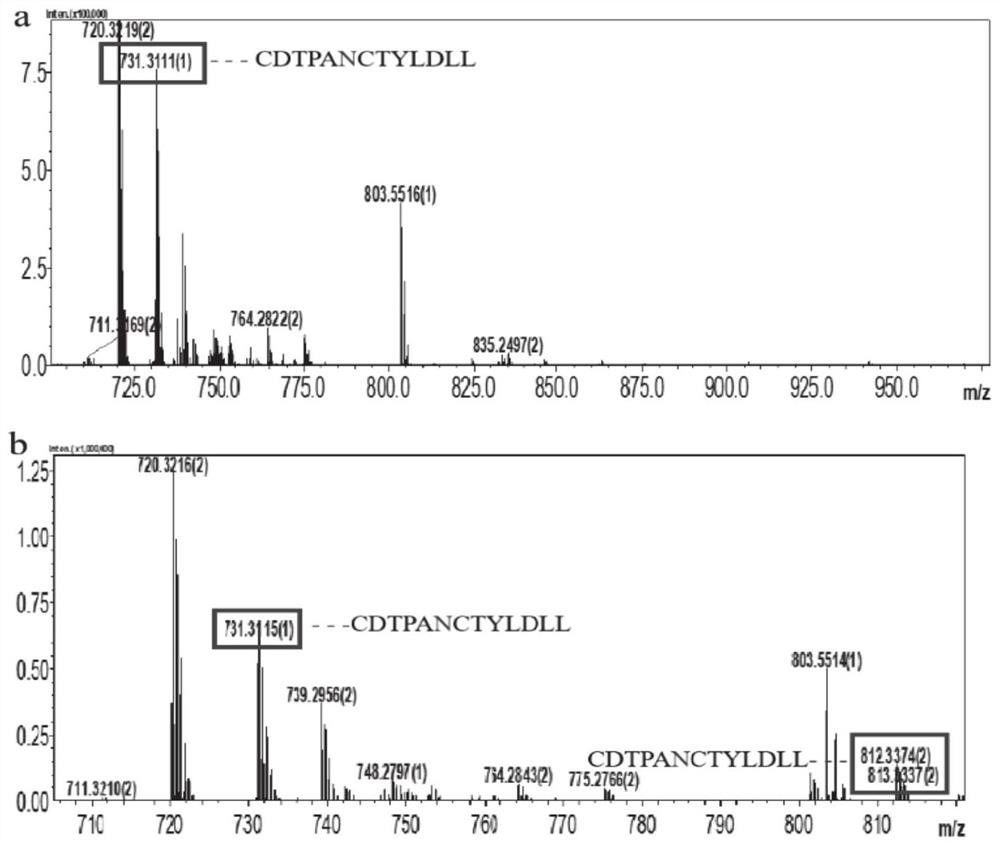
[0046] The enzyme activity results of F13 protein showed that the F13 mutant could not only glycosylate the natural substrate HMW1 fragment of wild-type NGT ( figure 2 ), and can glycosylate the fragment of diformyl peptidase CDTPANCTYLDLL ( image 3 ) and hemagglutinin fragment TLDDNGTMLFFK ( Figure 4 ) and IgG fragment REEQYNSTYRVVS ( Figure 5 ), indicating that the mut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com