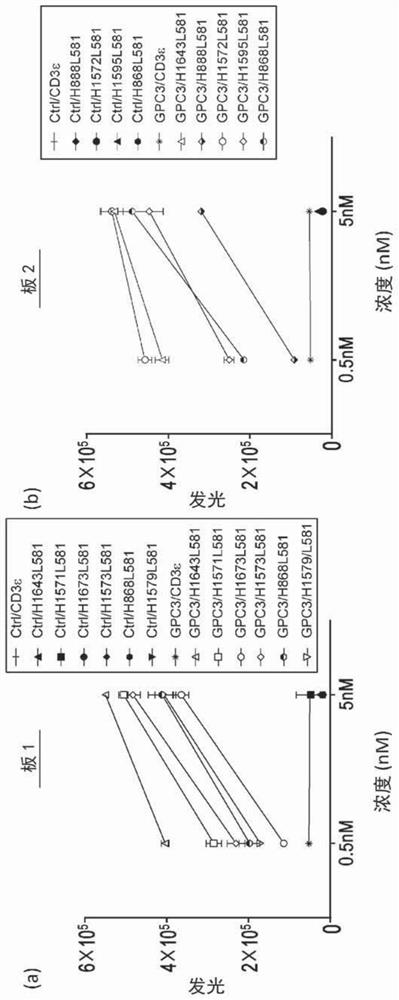
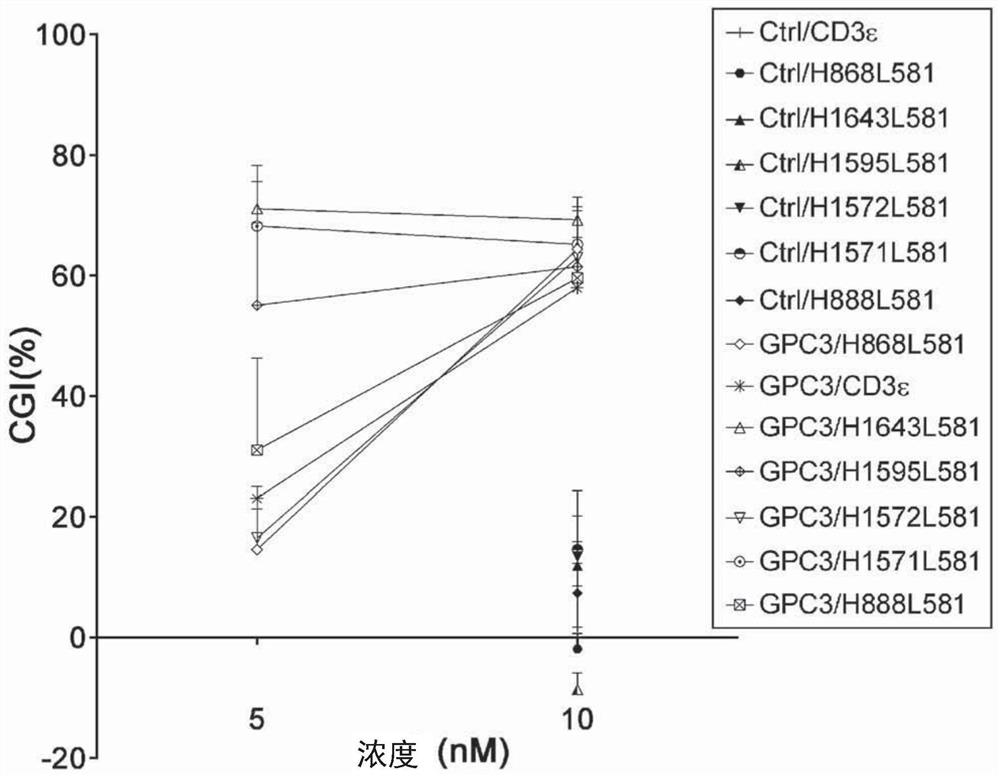
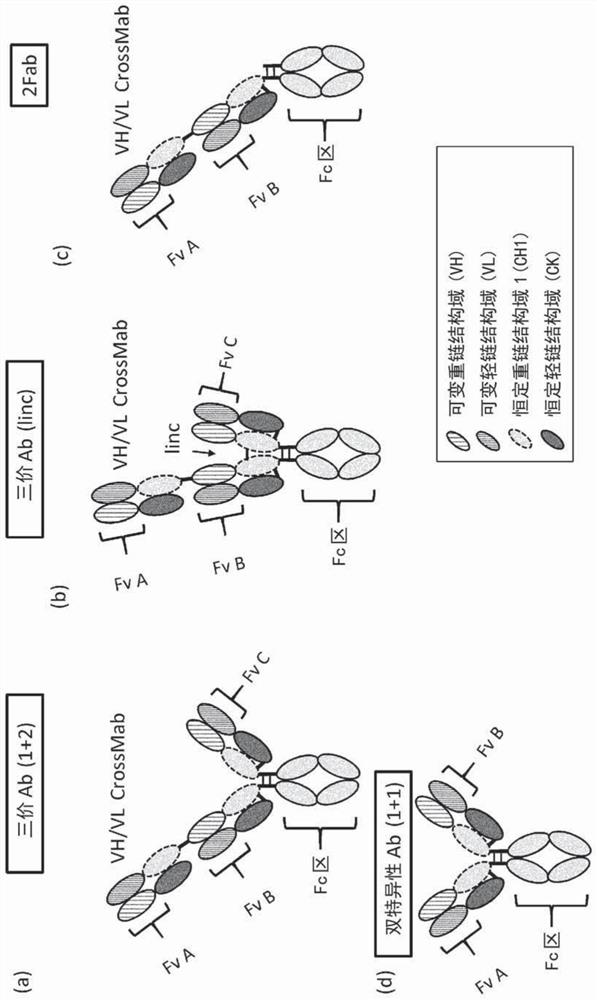
Antigen-binding molecule comprising altered antibody variable region
An antigen-binding molecule, antigen technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, hybrid immunoglobulin, anti-animal/human immunoglobulin, etc. progress, etc.
Pending Publication Date: 2021-08-13
CHUGAI PHARMA CO LTD
View PDF60 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the side effects of this CD137 agonist antibody are problematic both clinically and non-clinically due to its non-specific liver toxicity, and the development of agents has not progressed (Dubrot, Cancer Immunol. Immunother., 2010, 28, 512-22( NPL 22))
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 3
[0432] Example 3 - Anti-CD3 antibody that binds integrin and CD3, but not simultaneously.
Embodiment 4
[0433] Example 4 - Anti-CD3 antibody that binds TLR2 and CD3, but not simultaneously.
Embodiment 8
[0434] Example 8 - Anti-CD3 antibody that binds IgA and CD3, but not simultaneously.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
An antigen-binding molecule capable of binding to multiple different antigens (e.g., CD3 on T cells, and CD137 on T cells, NK cells, DC cells, and / or the like), but does not nonspecifically crosslink two or more immune cells such as T cells is provided. Such multispecific antigen-binding molecule is capable of modulating and / or activating an immune response while circumventing the cross-linking between different cells (e.g., different T cells) resulting from the binding of a conventional multispecific antigen-binding molecule to antigens expressed on the different cells, which is considered to be responsible for adverse reactions when the multispecific antigen-binding molecule is used as a drug.
Description
technical field [0001] The invention provides antigen-binding molecules capable of modulating and / or activating an immune response; pharmaceutical compositions comprising any of said antigen-binding molecules; and methods of producing said antigen-binding molecules. Background technique [0002] Antibodies have high stability in plasma and produce few adverse reactions, thus attracting attention as drugs (Nat. Biotechnol. (2005) 23, 1073-1078 (NPL 1) and Eur J Pharm Biopharm. (2005) 59 (3), 389-396 (NPL 2)). Antibodies not only have antigen-binding effects and agonist or antagonist effects, but also induce effector cell-mediated cytotoxic activity (also known as effector function), such as ADCC (antibody-dependent cellular cytotoxicity), ADCP (antibody-dependent cellular phagocytosis) effect) or CDC (complement-dependent cytotoxicity). In particular, antibodies of the IgGl subclass exhibit effector functions against cancer cells. Accordingly, a large number of antibody dr...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07K16/46C12N15/09C12P21/08C07K16/28
CPCC07K16/2809C07K16/2878C07K16/303C07K2317/55C07K2317/31C07K2317/35C07K2317/92C07K2317/56C07K2317/75C07K2317/94C07K2317/73C12N15/1034C07K2317/522C07K2317/565C07K2317/526C07K16/468C07K16/2896C07K2317/53C07K2317/71
Inventor 井川智之冯舒何菽文白岩宙丈
Owner CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com