Method and apparatus for refilling fuel to electrochemical power source
An electrochemical and power supply technology, applied in electrochemical generators, transfer electrolyte devices, fuel cell-type half-cells and primary battery-type half-cells, etc., can solve the problems of not 100% utilization of zinc, user exposure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
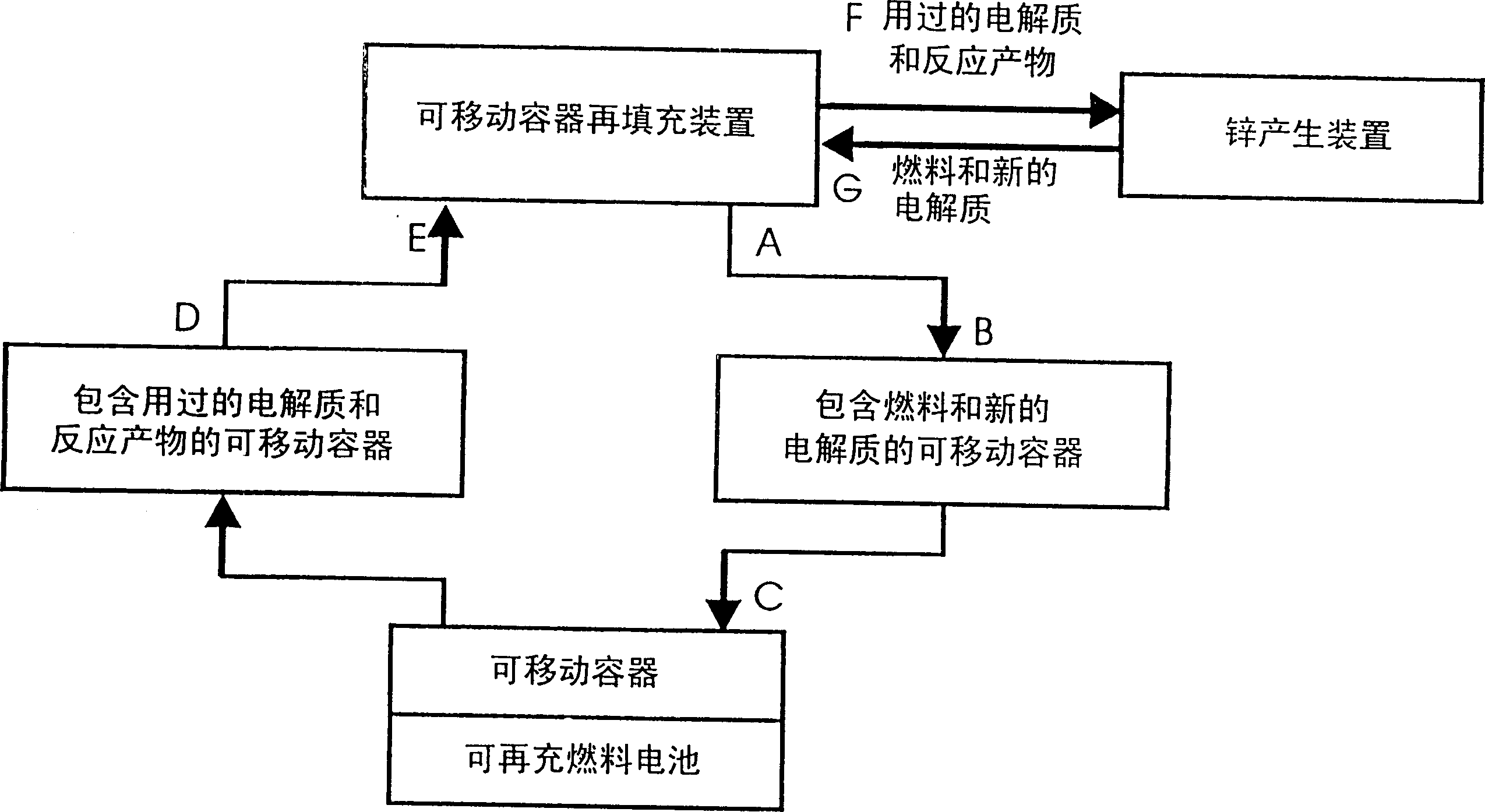
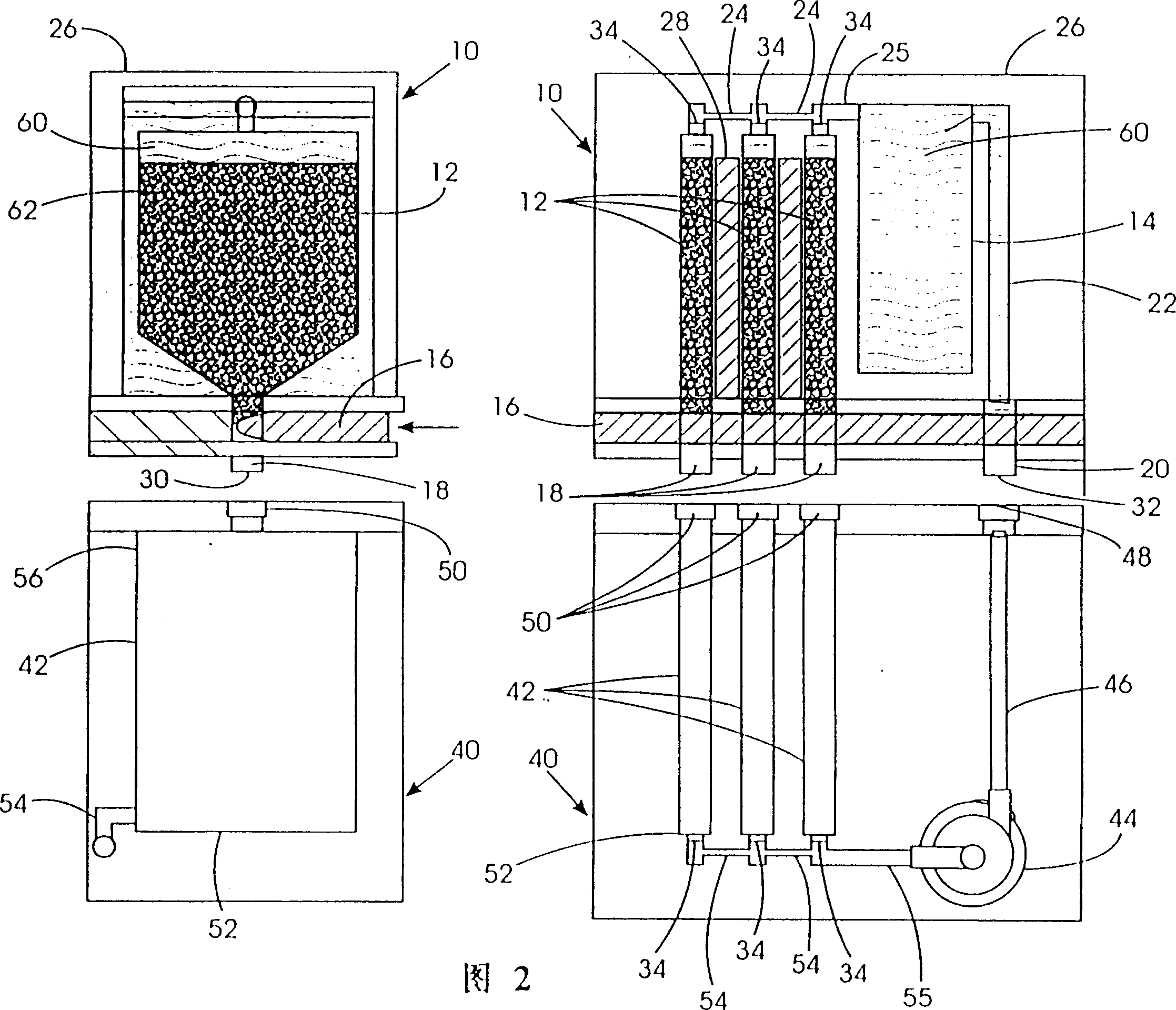
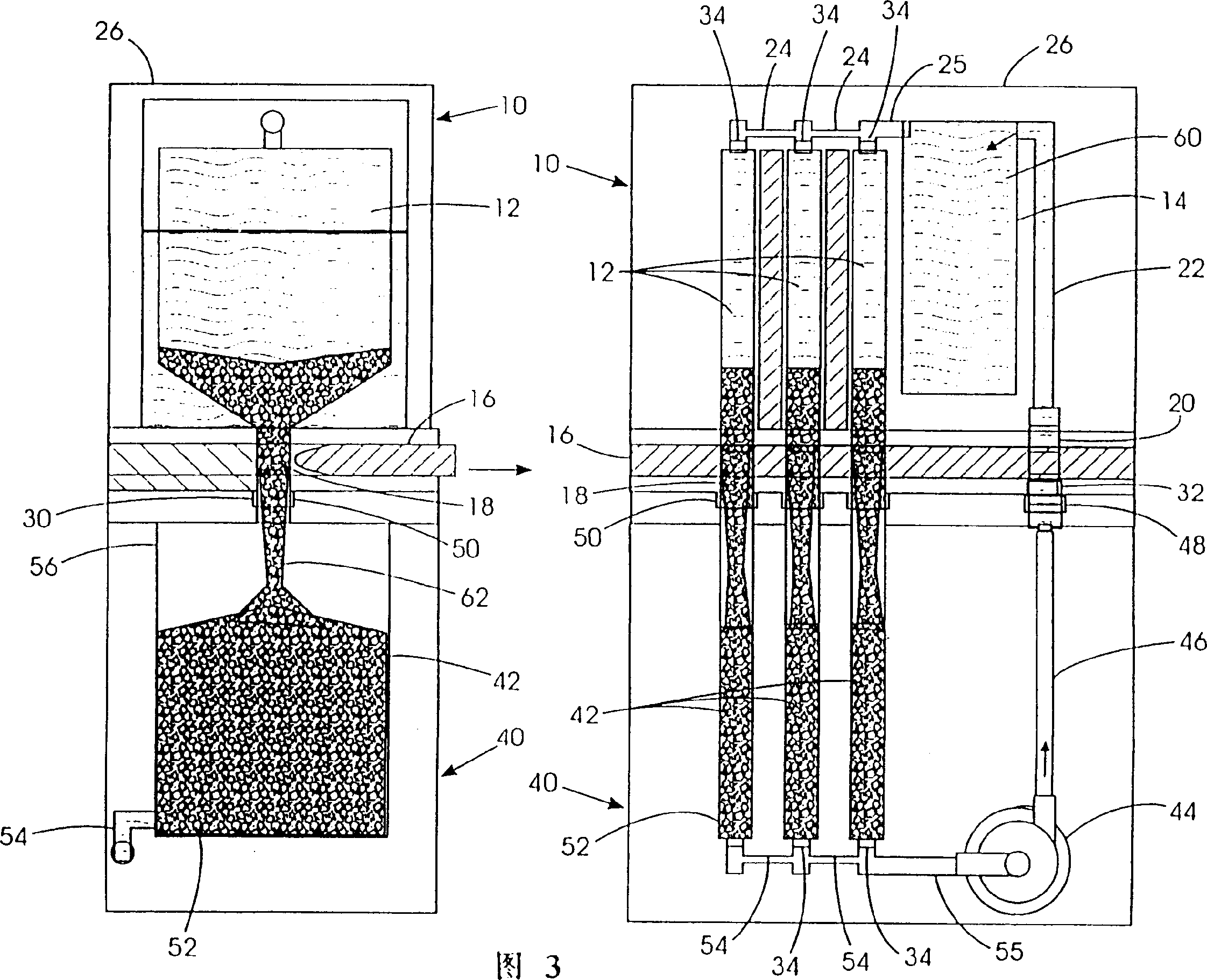
[0032] Disclosed herein is a system capable of filling electrochemically active fuel particles (such as zinc particles) and electrolyte (such as potassium hydroxide) from removable containers into a reproducible battery cell with specific electrodes utilizing anodic processes. In the fuel cell. Also provided is a cyclic process of recycling spent electrolyte and reaction products from a discharged battery cell back to a removable container as a filling process to remove electrochemically active particles and electrolyte from a device for storing and forming electrochemically active fuel particles Fill into removable containers. The reaction products from the spent electrolyte and discharged cells from the removable container are conveyed to means for storing and regenerating the reaction products.
[0033] One or more removable containers may be attached or inserted into an electrochemical device, such as a metal / air refuelable cell. Electrochemically active fuel particles i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com