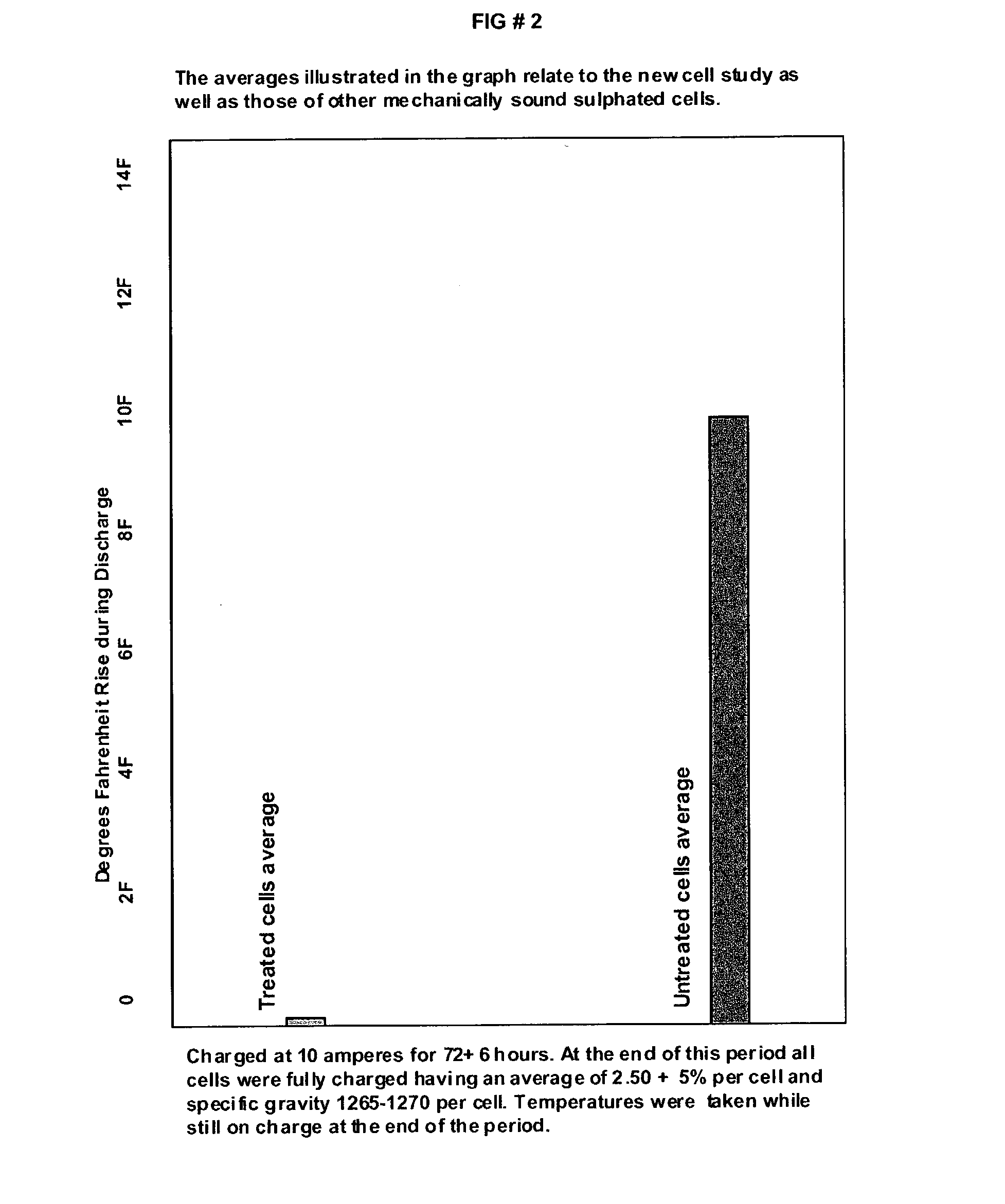
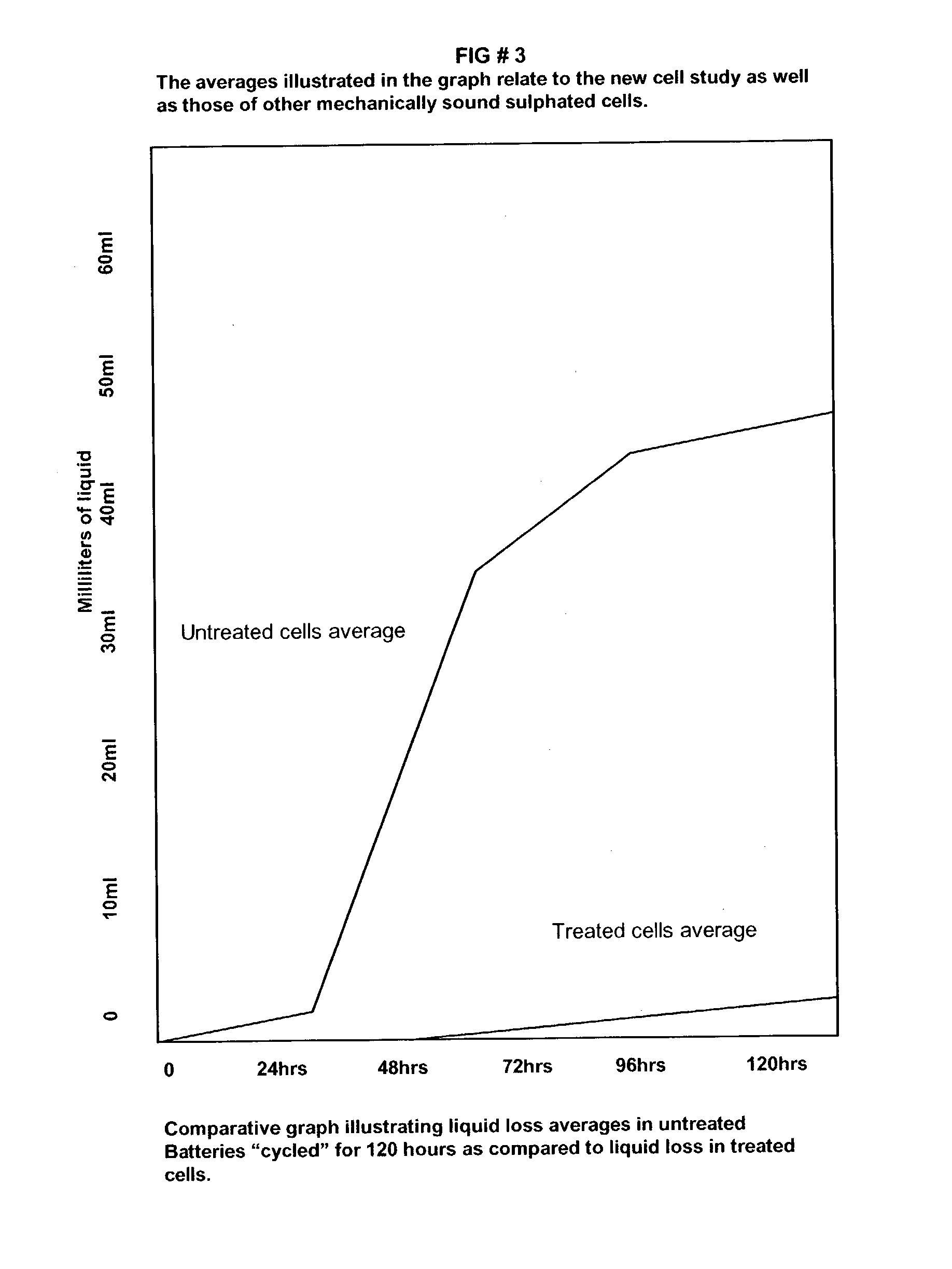
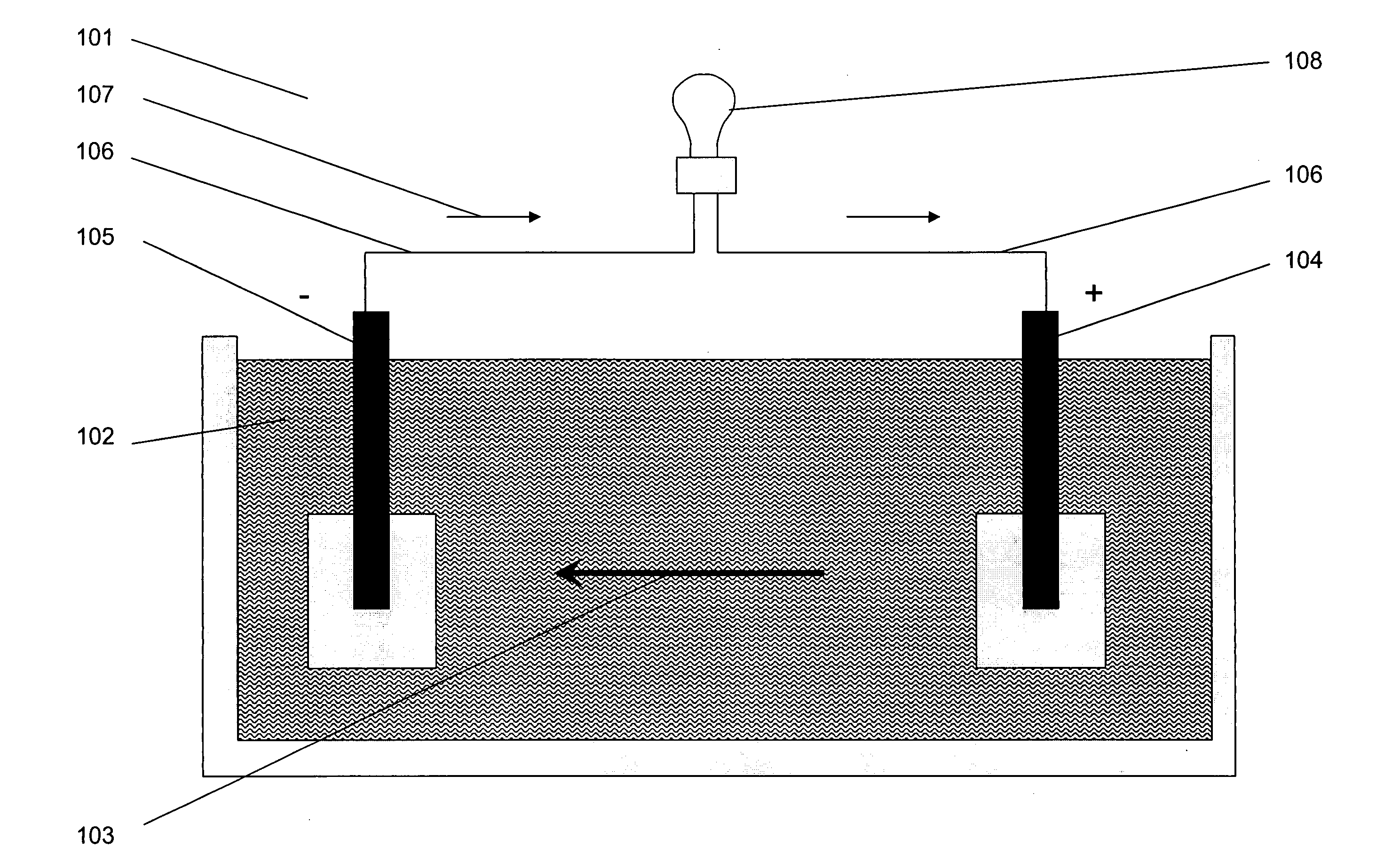
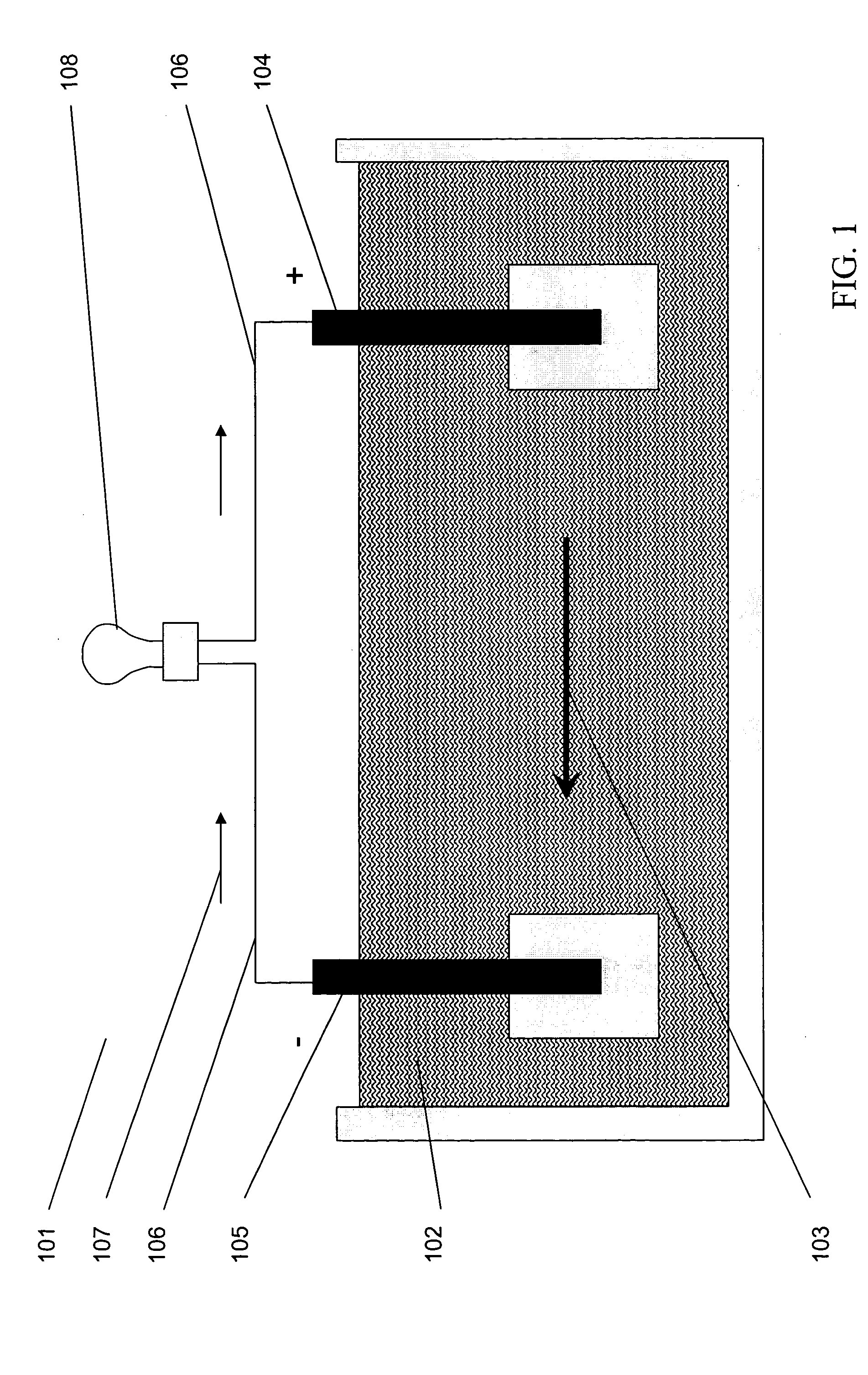
Patents
Literature
48results about "Reactants/electrolytes regeneration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
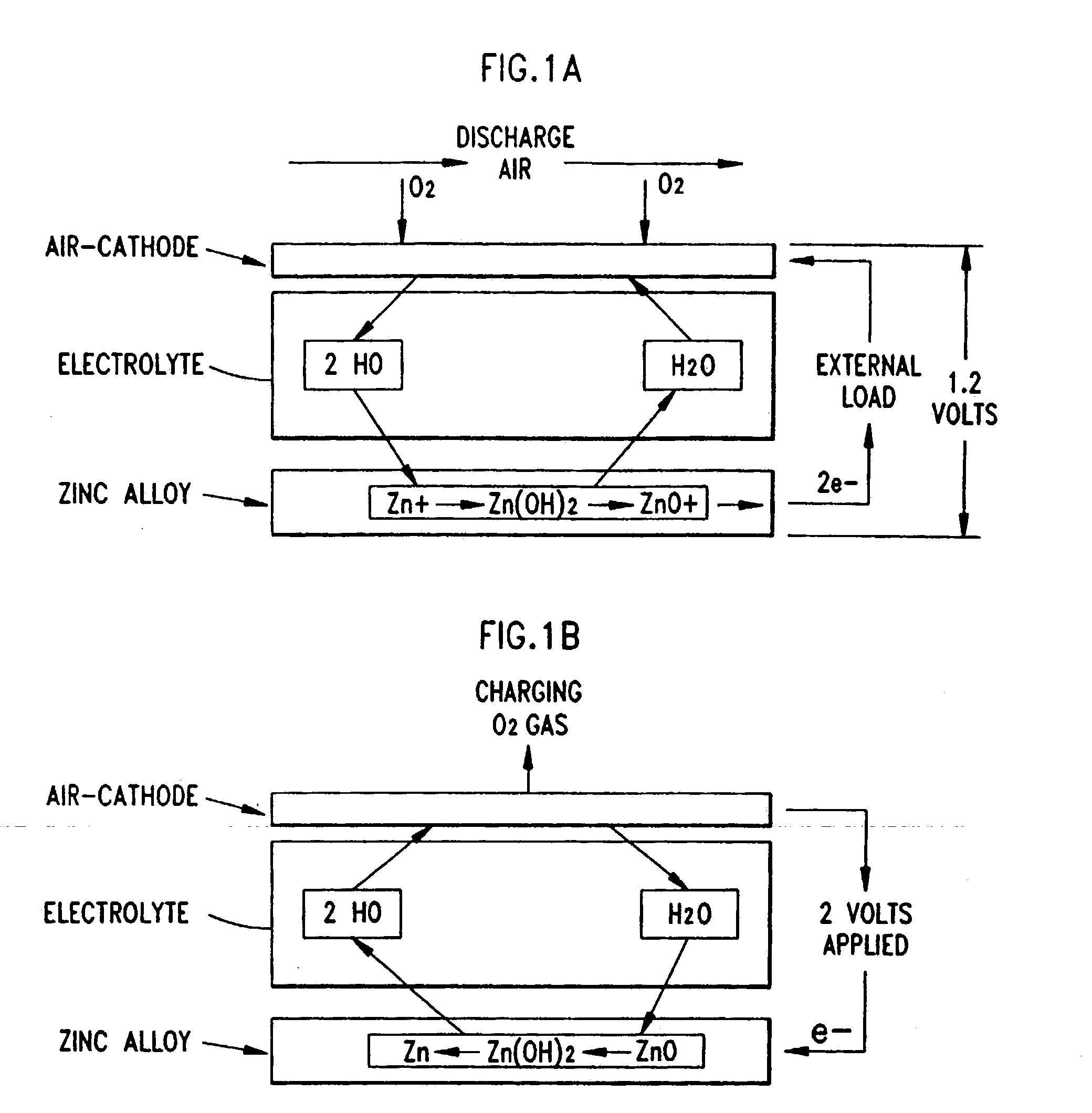
Air battery system
InactiveUS20100151336A1Avoid internal resistanceFuel and primary cellsFuel and secondary cellsHigh rateInternal resistance
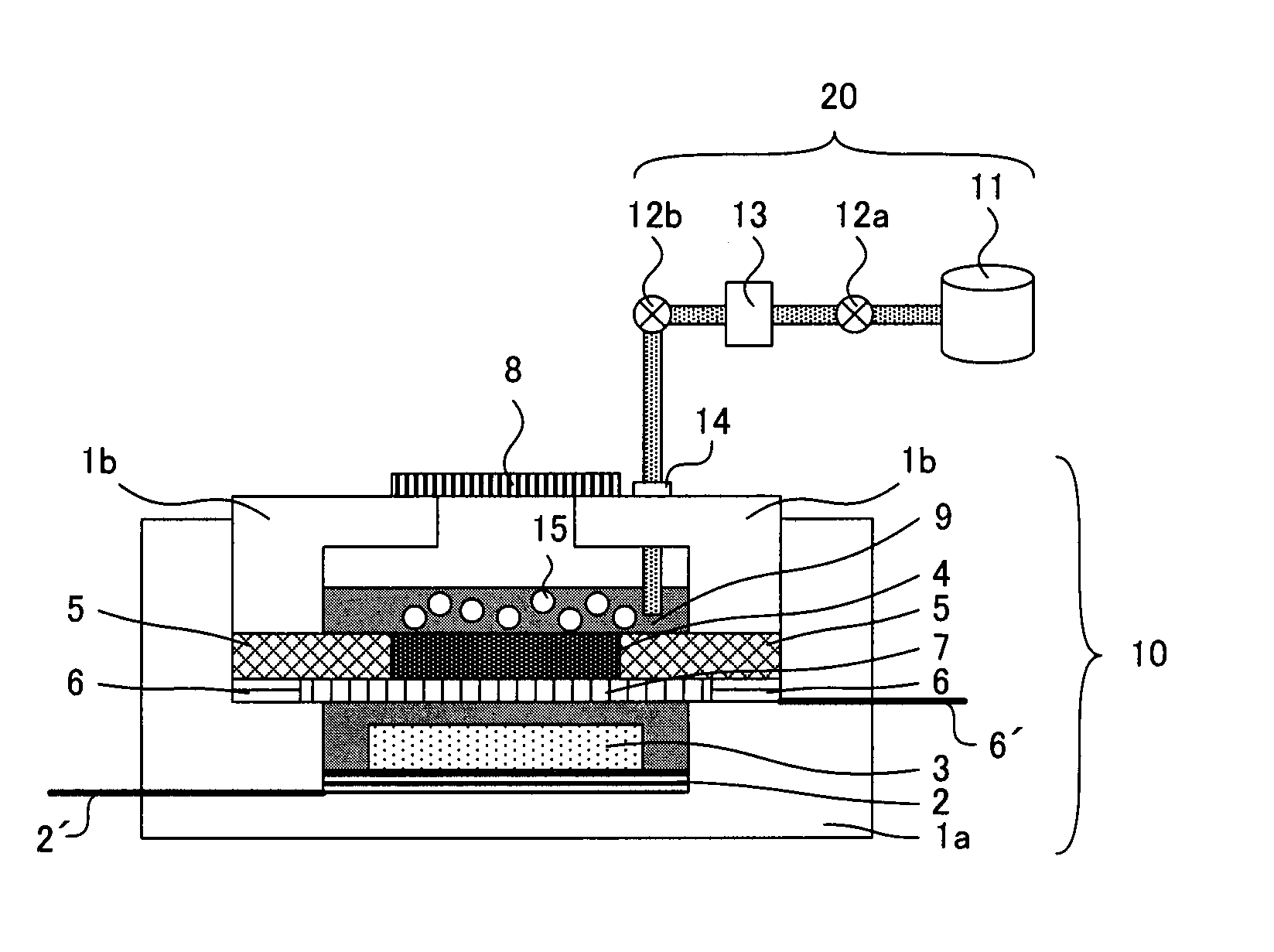
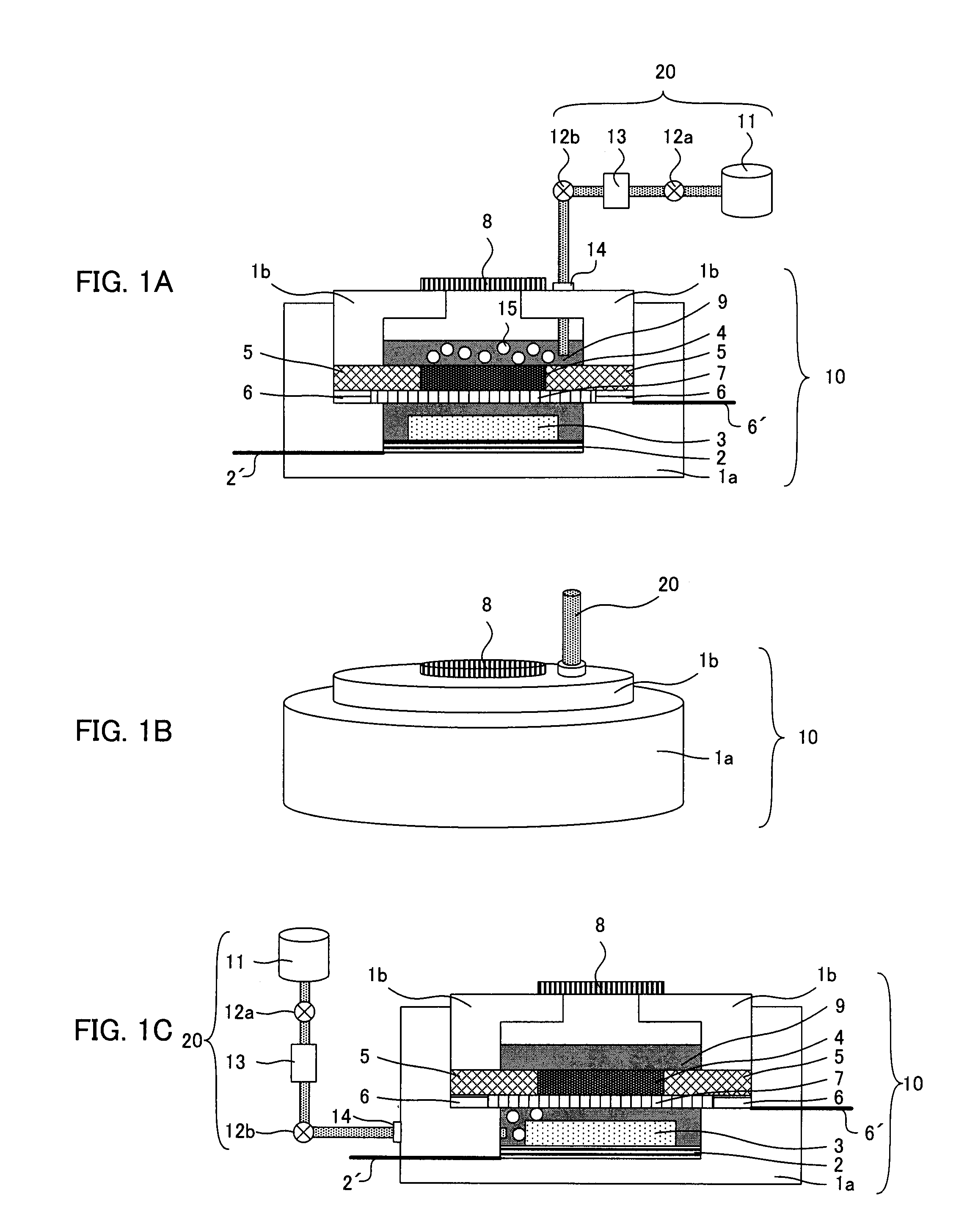
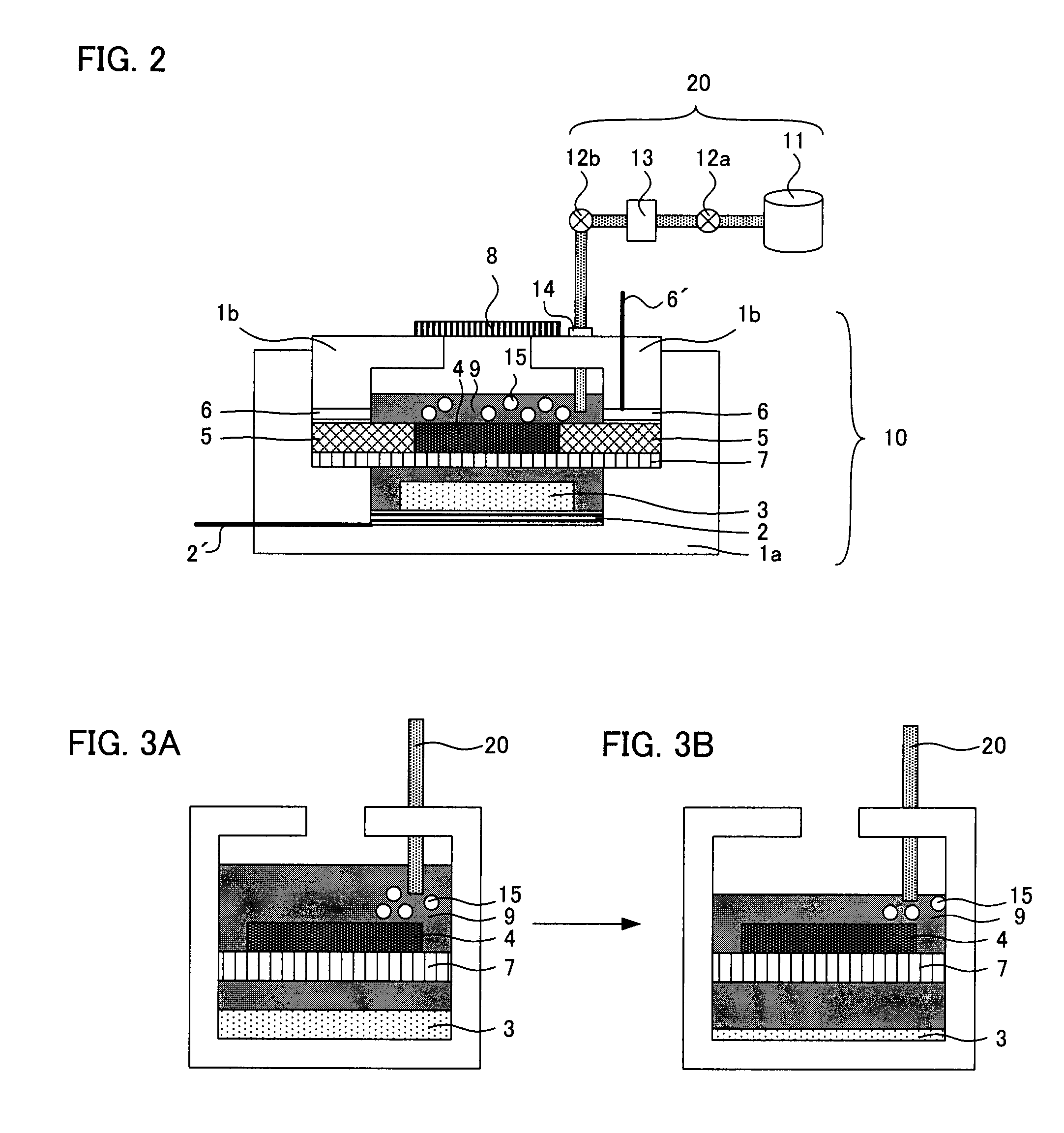
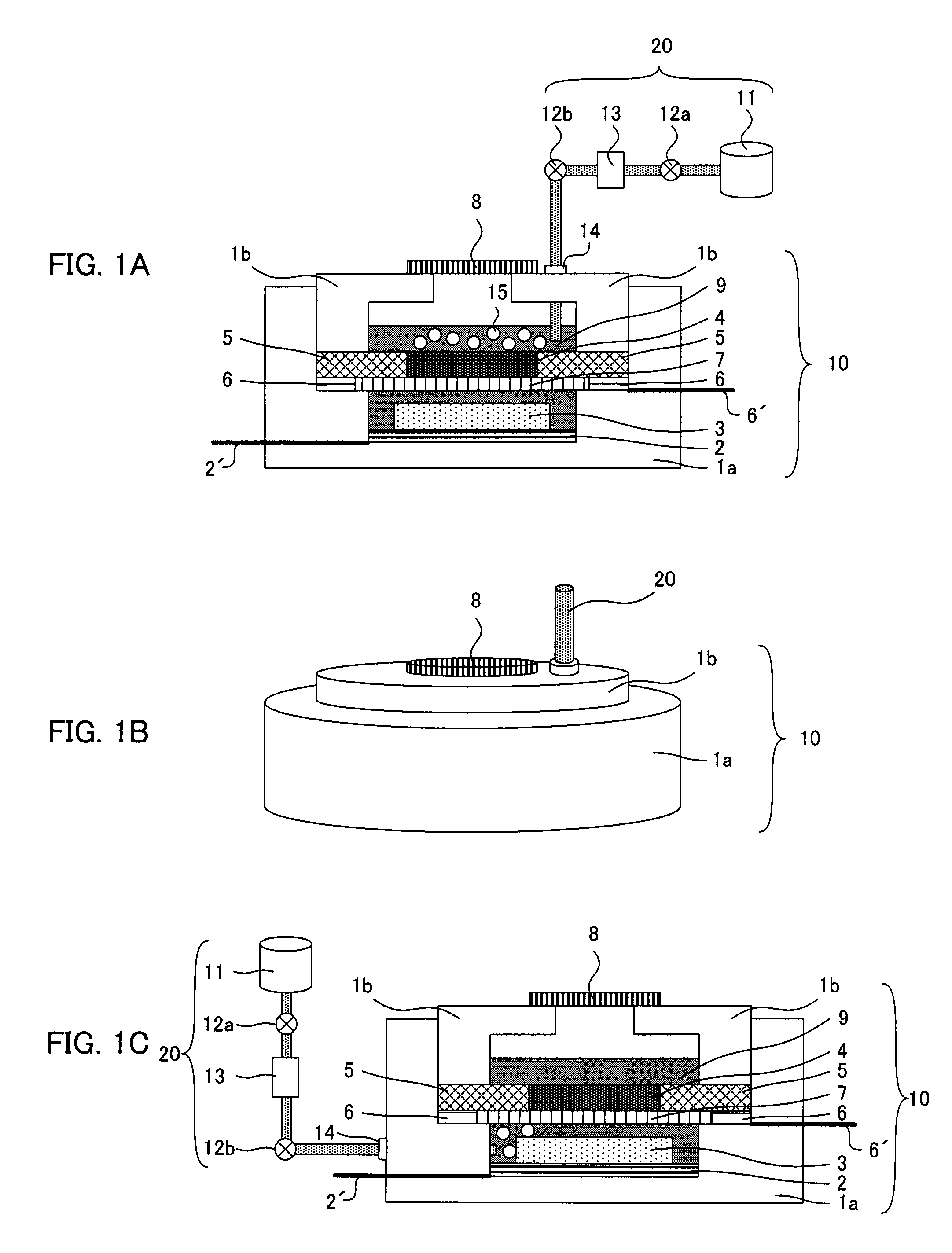
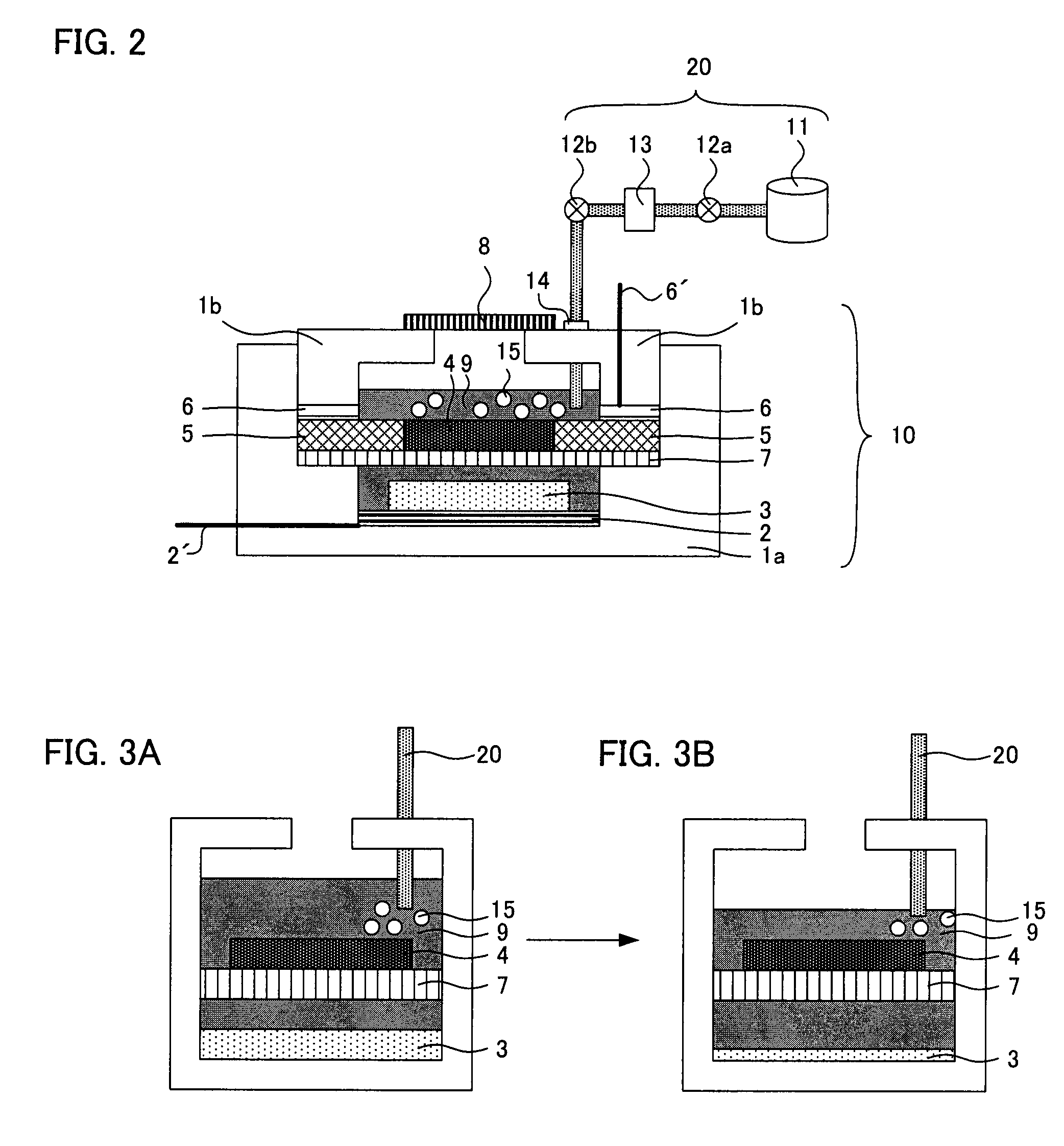
A main object of the present invention is to provide an air battery system which can restrain the internal resistance caused by the shortage of liquid electrolyte from increasing and which can carry out a high-rate discharge. The present invention resolves the above-mentioned object by providing an air battery system comprising: an air battery cell which contains an air cathode, an anode, and a separator; and an oxygen gas supply means for supplying an oxygen gas by bubbling to a liquid electrolyte, characterized in that the air cathode further contains an air cathode layer containing a conductive material, and an air cathode current collector for collecting current of the air cathode layer; the anode further contains an anode layer containing an anode active material which stores and releases a metal ion, and an anode current collector for collecting current of the anode layer; and the separator is provided between the air cathode layer and the anode layer, and characterized in that the air cathode layer and the anode layer are constantly filled with the liquid electrolyte at a time of a change in a volume of the electrode caused by a discharge or a discharge and charge.
Owner:TOYOTA JIDOSHA KK
Battery
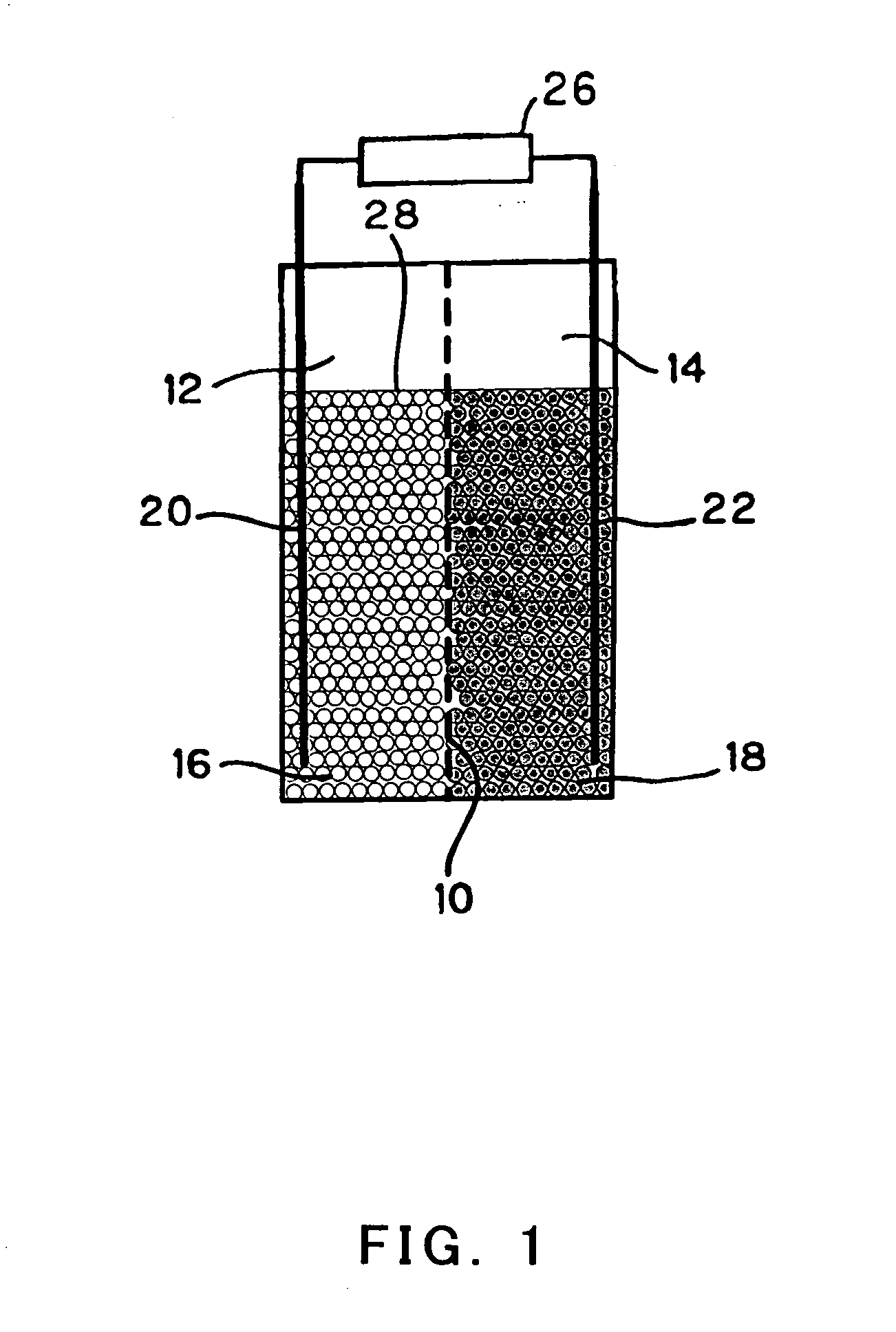
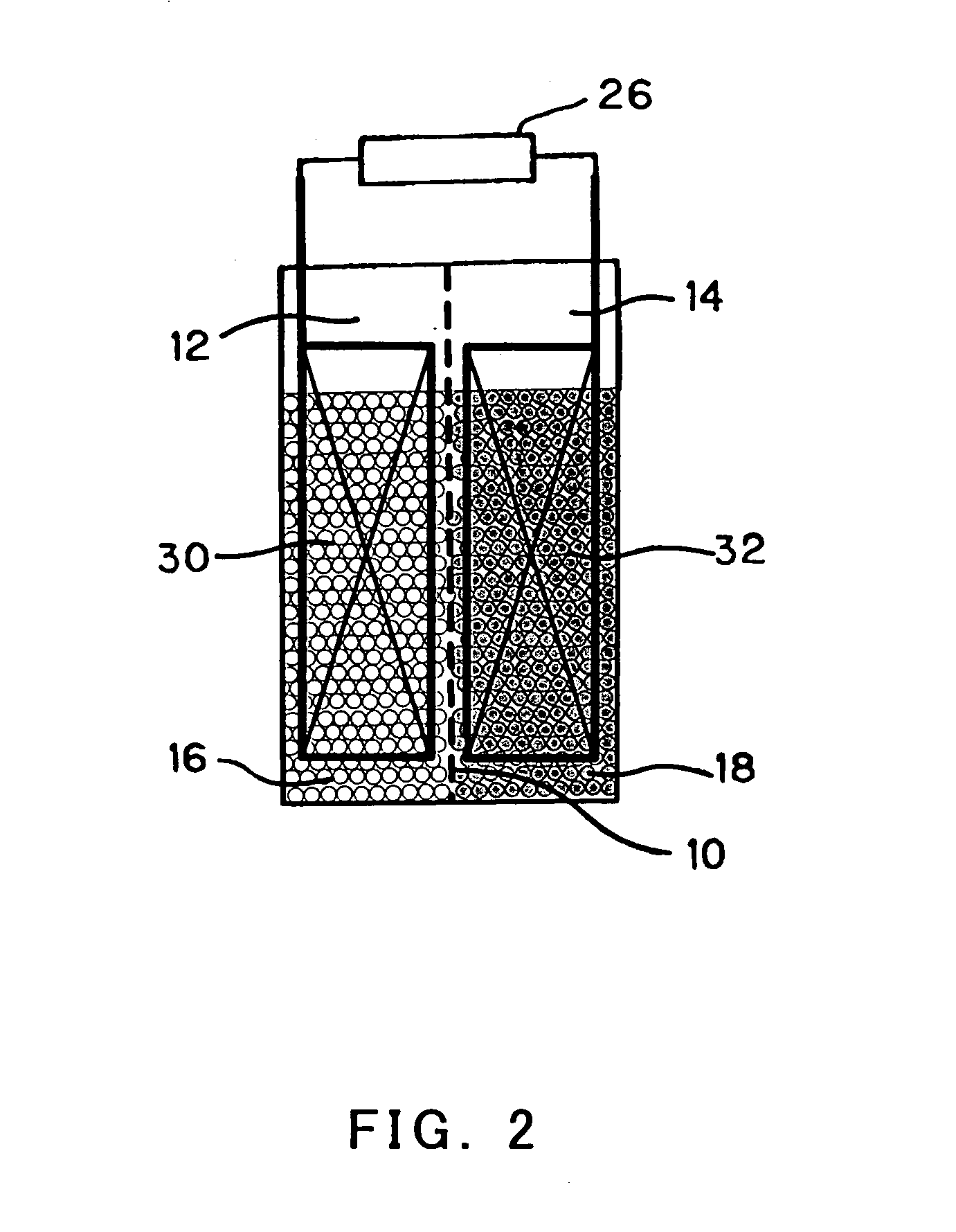
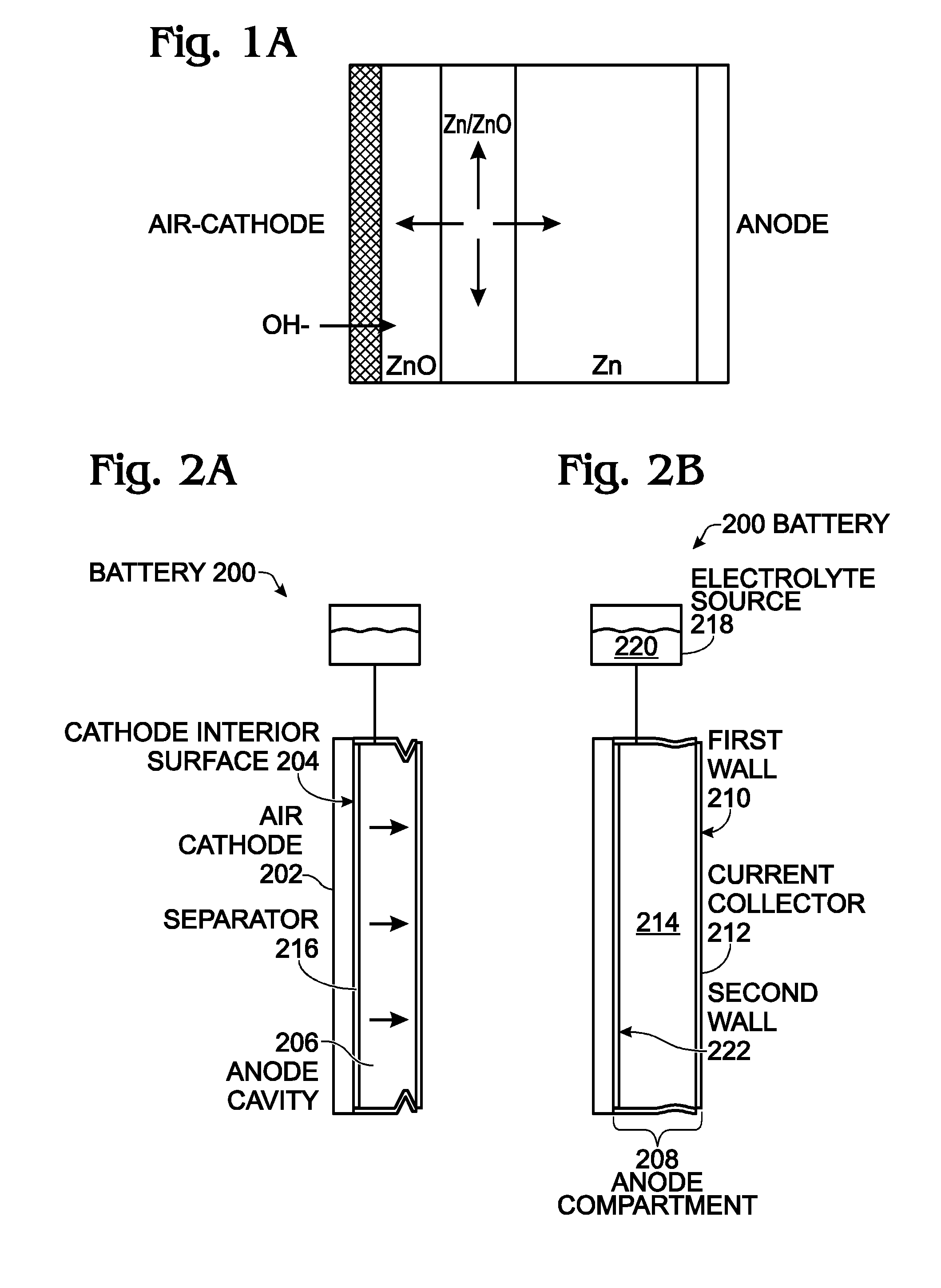
InactiveUS20050175890A1Production cost advantageIncrease capacityPrimary cell to battery groupingMoving electrode arrangementsEngineeringIon
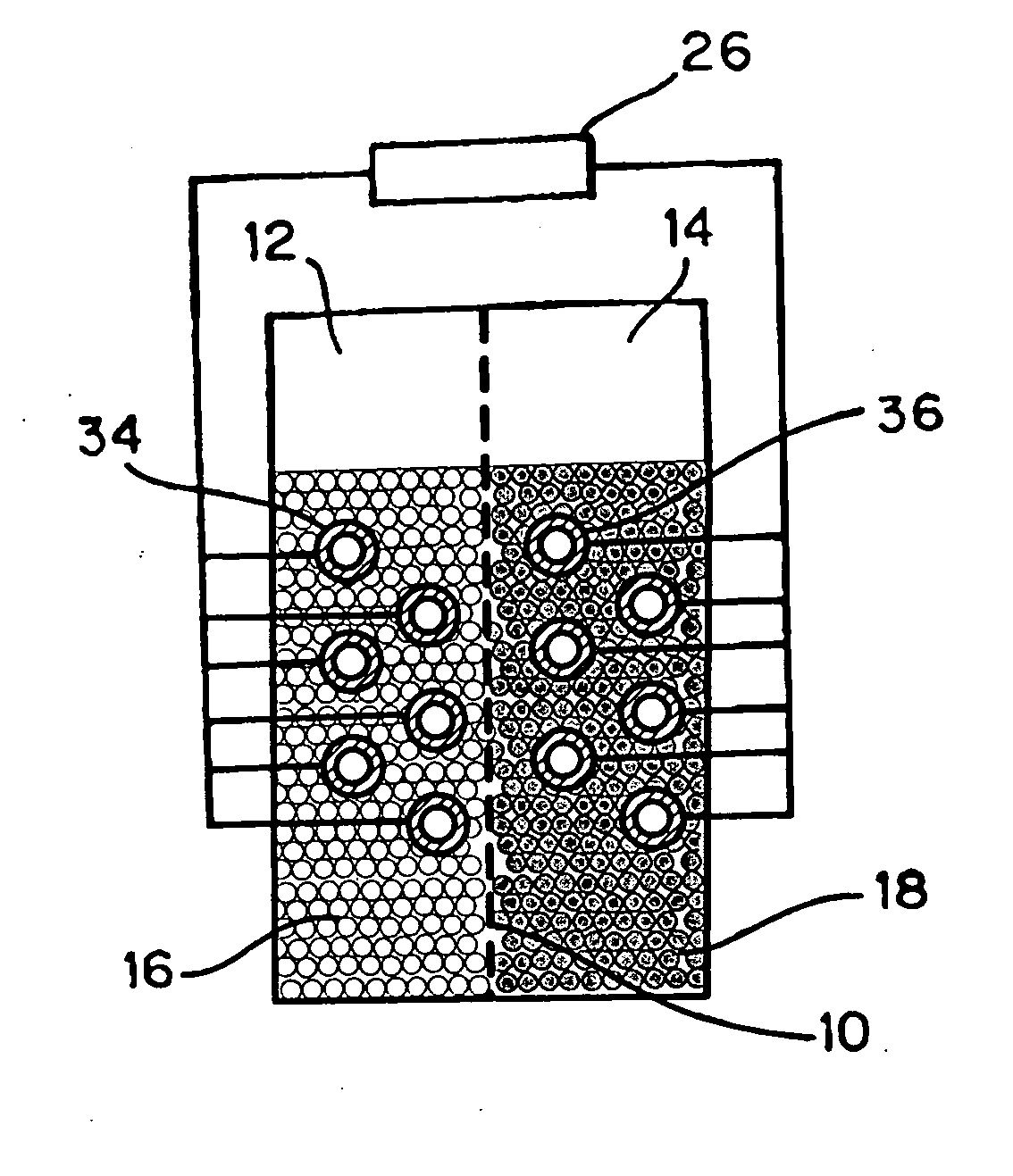
Anode active material particles and an electrolytic solution 16 are filled in an anode cell 12 as one of two vessels connected to each other with an ion-permeable separator 10 interposed therebetween, and cathode active material particles and an electrolytic solution 18 are filled in a cathode cell 14 as the other vessel. Electrically conductive current collectors 20 and 22 are provided in contact with the active material particles within the two vessels. The active material particles form fixed layers.
Owner:KAWASAKI HEAVY IND LTD
Battery
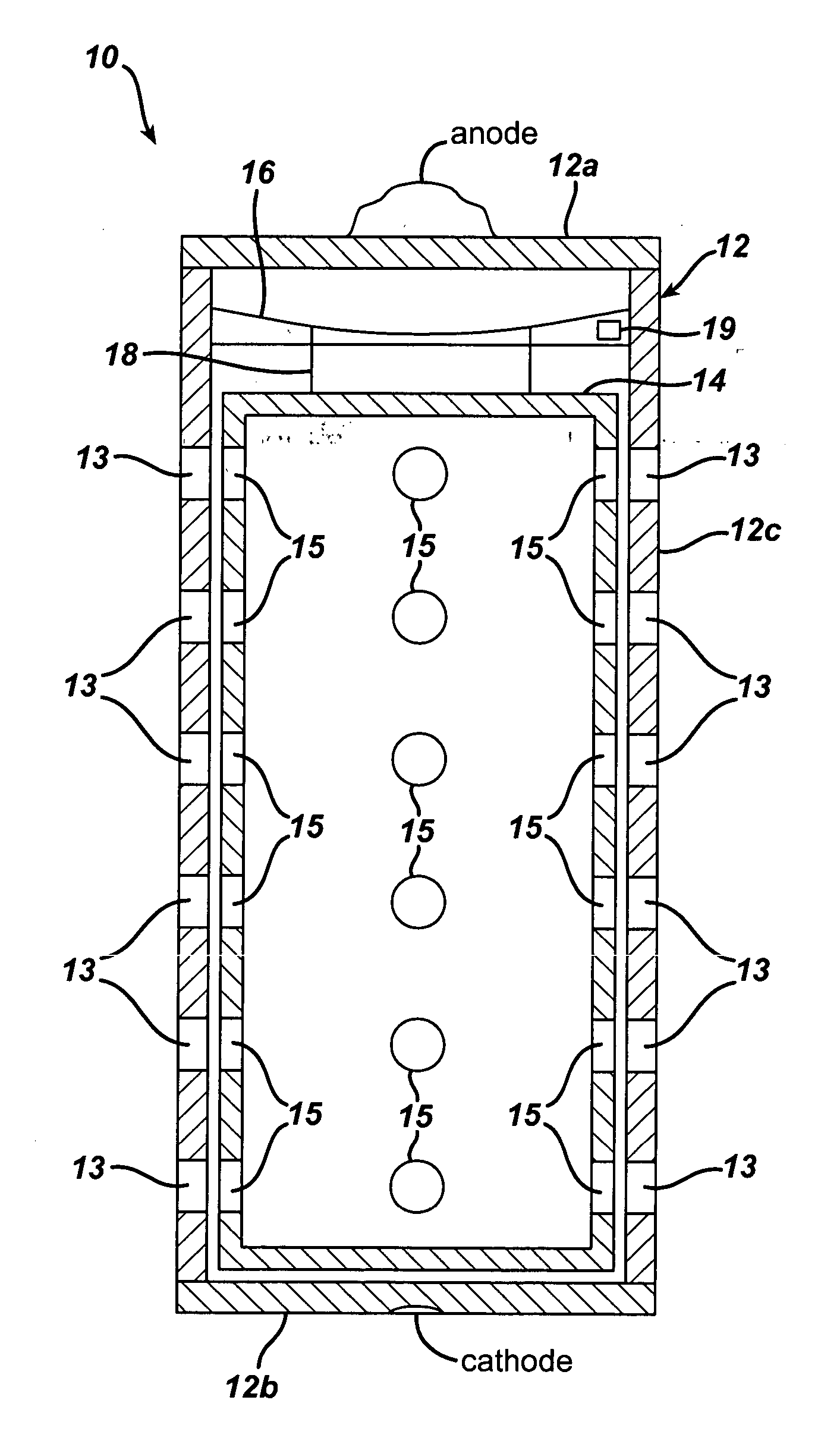
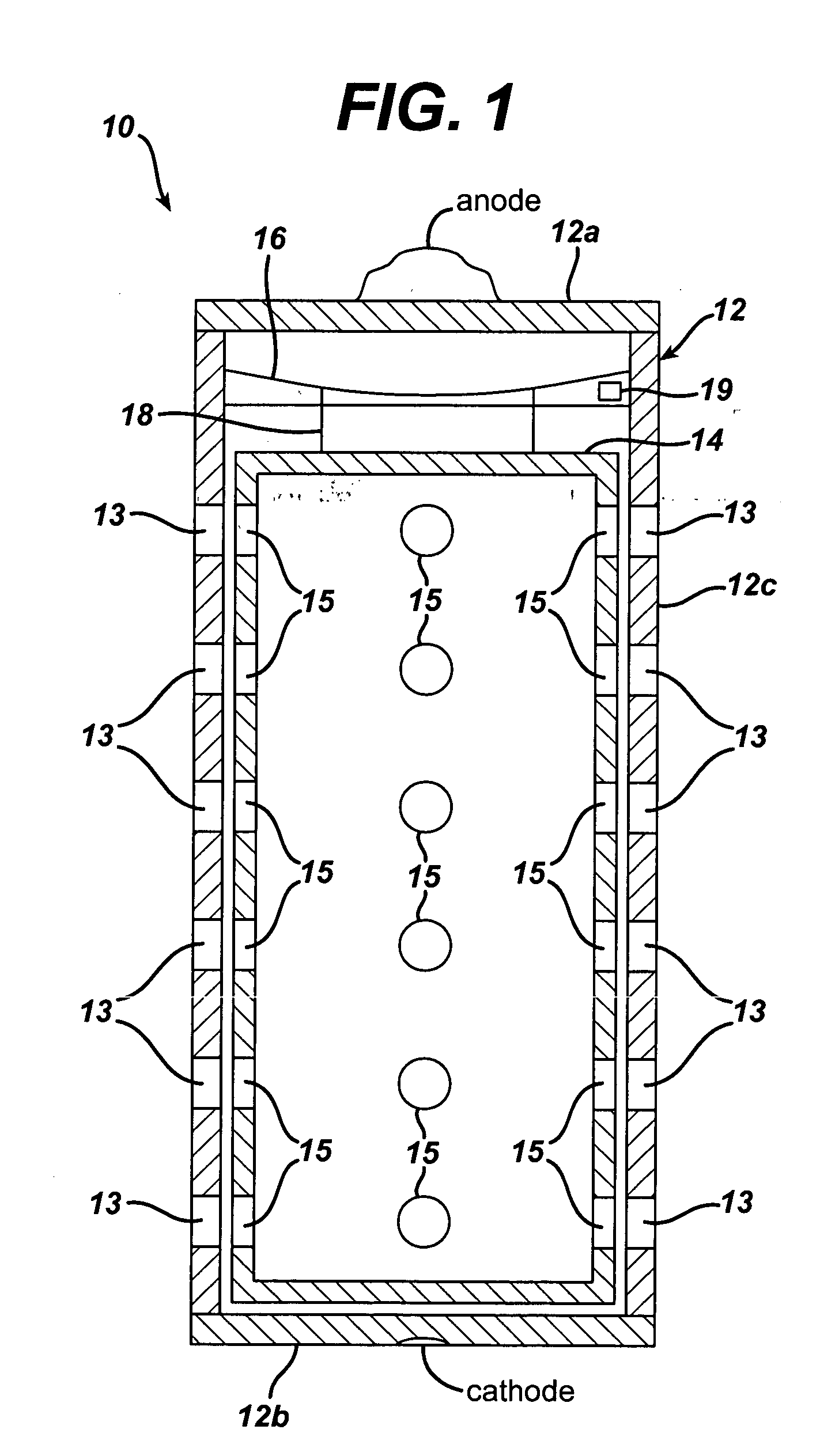
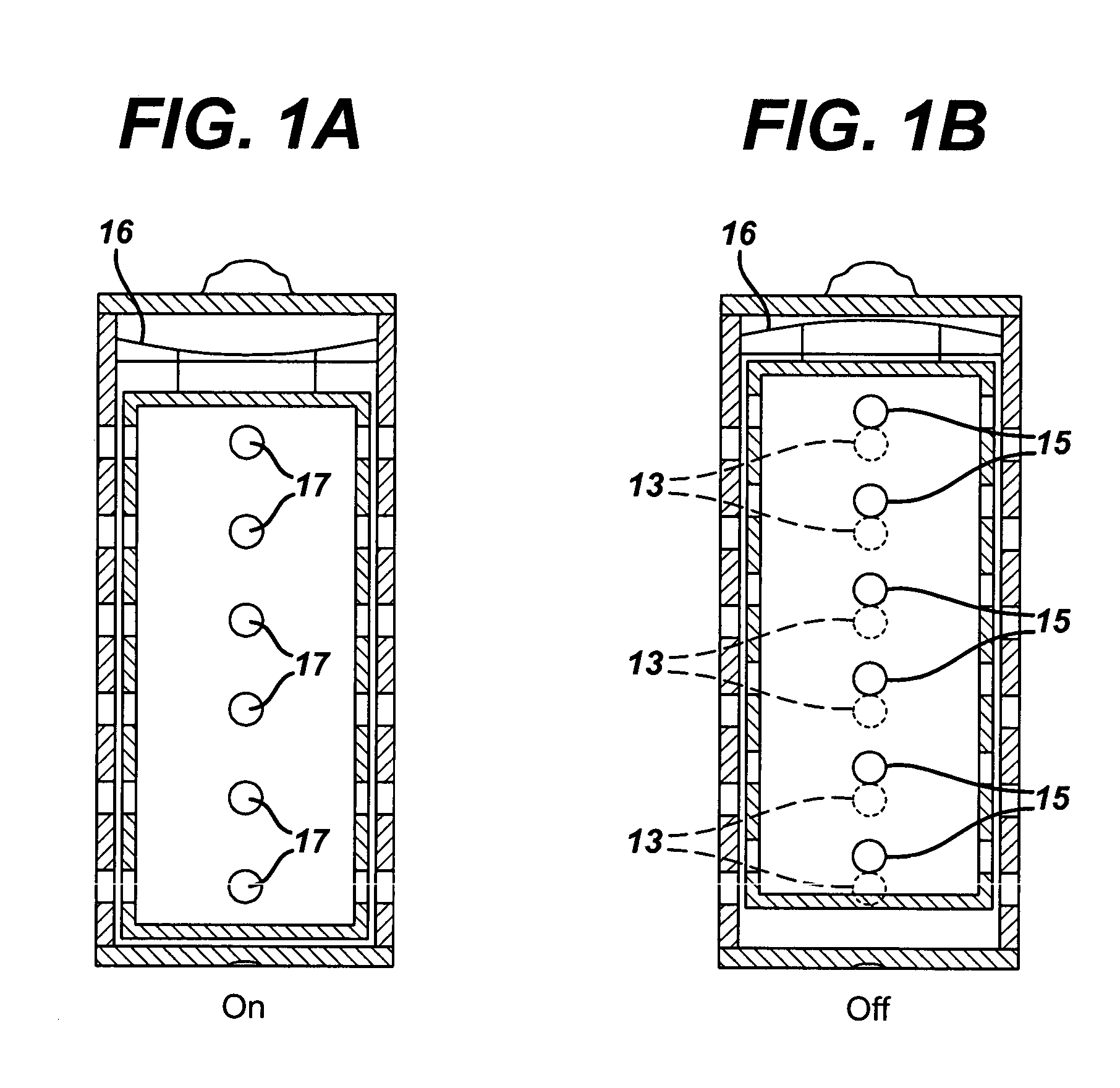
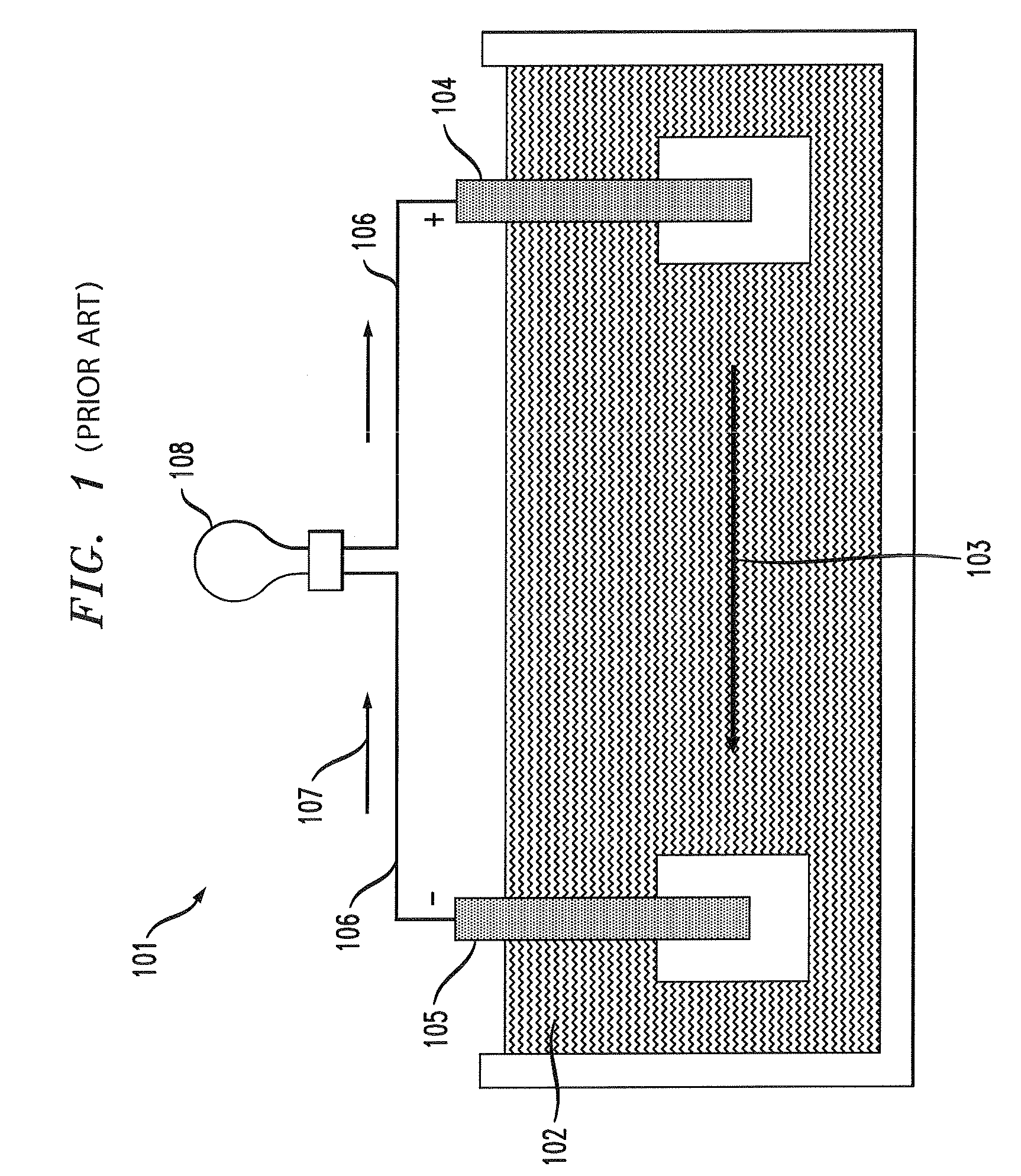
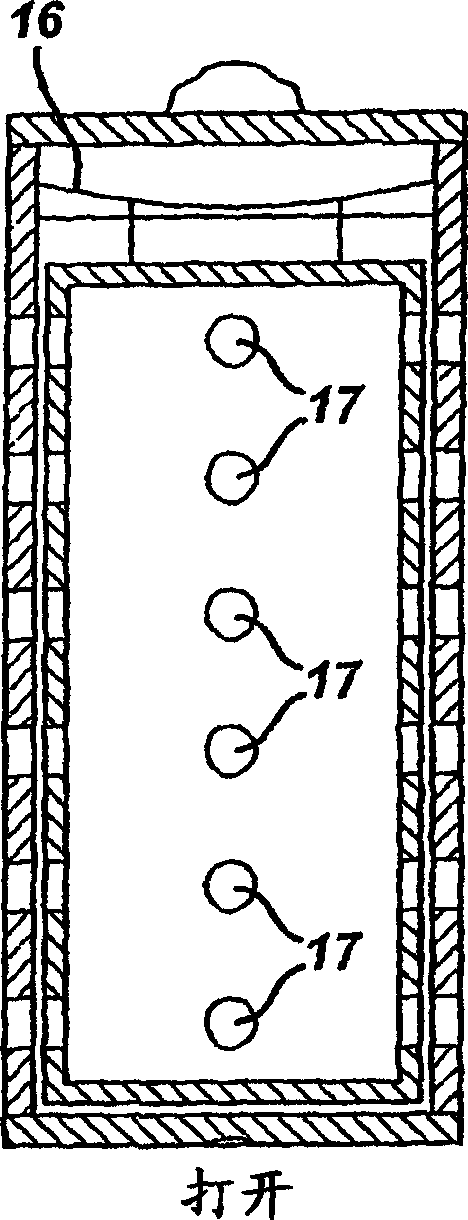
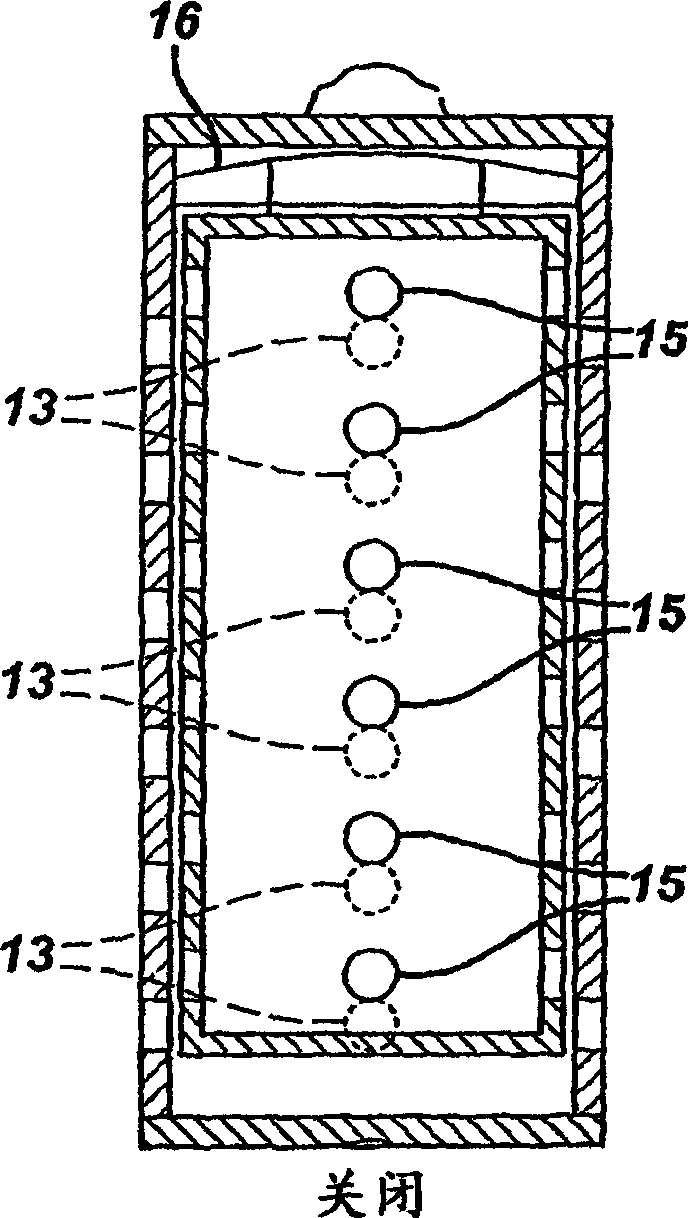
ActiveUS20050058887A1Extended service lifeLimited service lifeFuel and primary cellsOperating means/releasing devices for valvesElectrochemical responseChemical reaction
A battery includes a battery can housing an cell that supplies electrical energy at terminals of the cell by an electro-chemical reaction with oxygen, the can including, a first member having at least one hole that is exposed to air; and a second member. The battery also includes a mechanism coupled to one of the first and second members to move the one of the first and second members such that when current is drawn from the battery, the opening in the member allows air to pass into the battery, and to move the one of the first and second members such that when current is not drawn from the battery, the opening in the member is not in registration to inhibit air to pass into the battery. The battery also includes a circuit to control the mechanism. In one embodiment the circuit monitors levels of O2 in a air plenum that surrounds the cell. The circuit to monitor levels of O2 in the air plenum includes a florescent detector / sensor that senses and responds to changes in O2 in the plenum by using the “quenching effect” of oxygen on a fluorescent material
Owner:DURACELL U S OPERATIONS
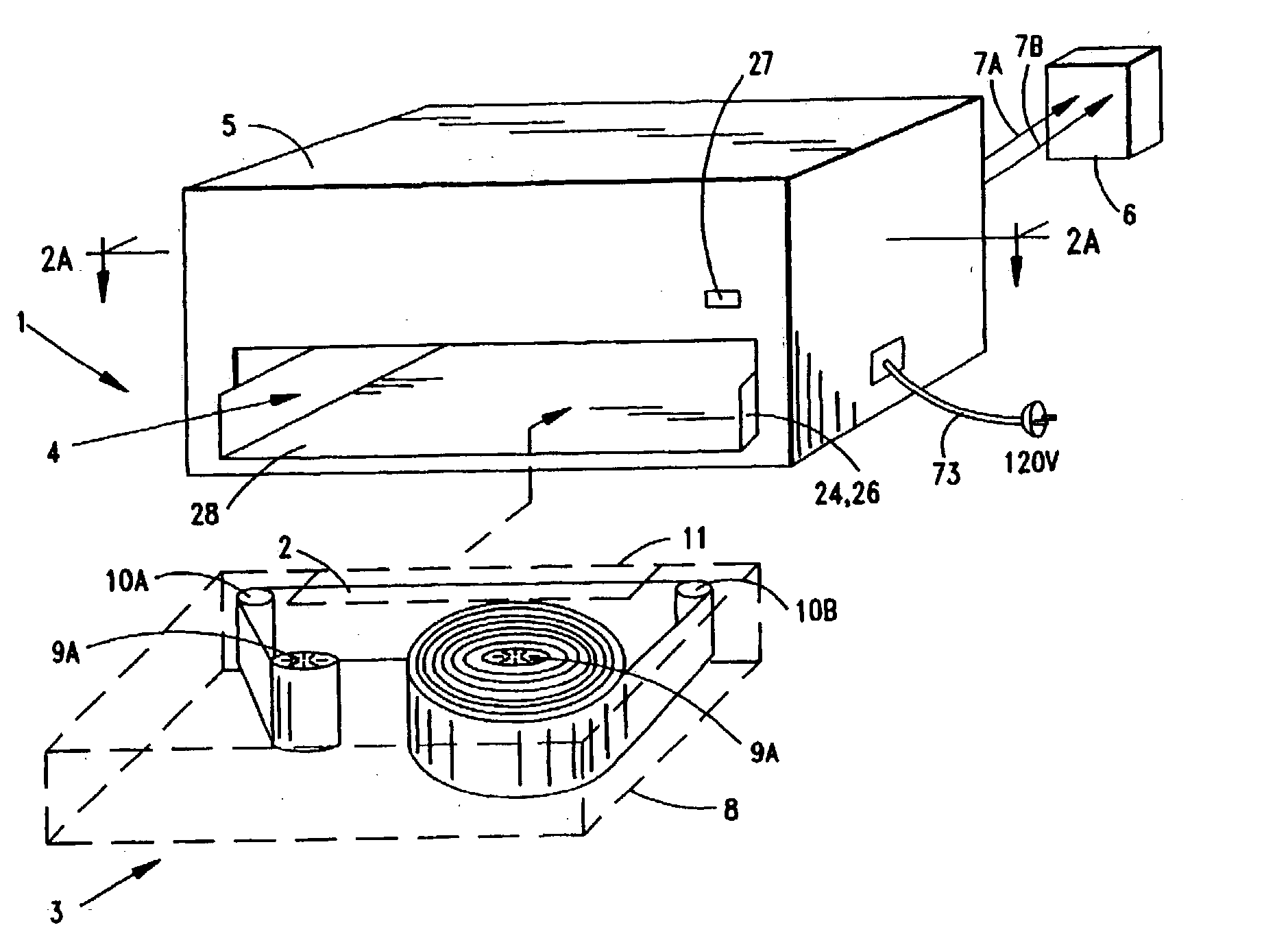
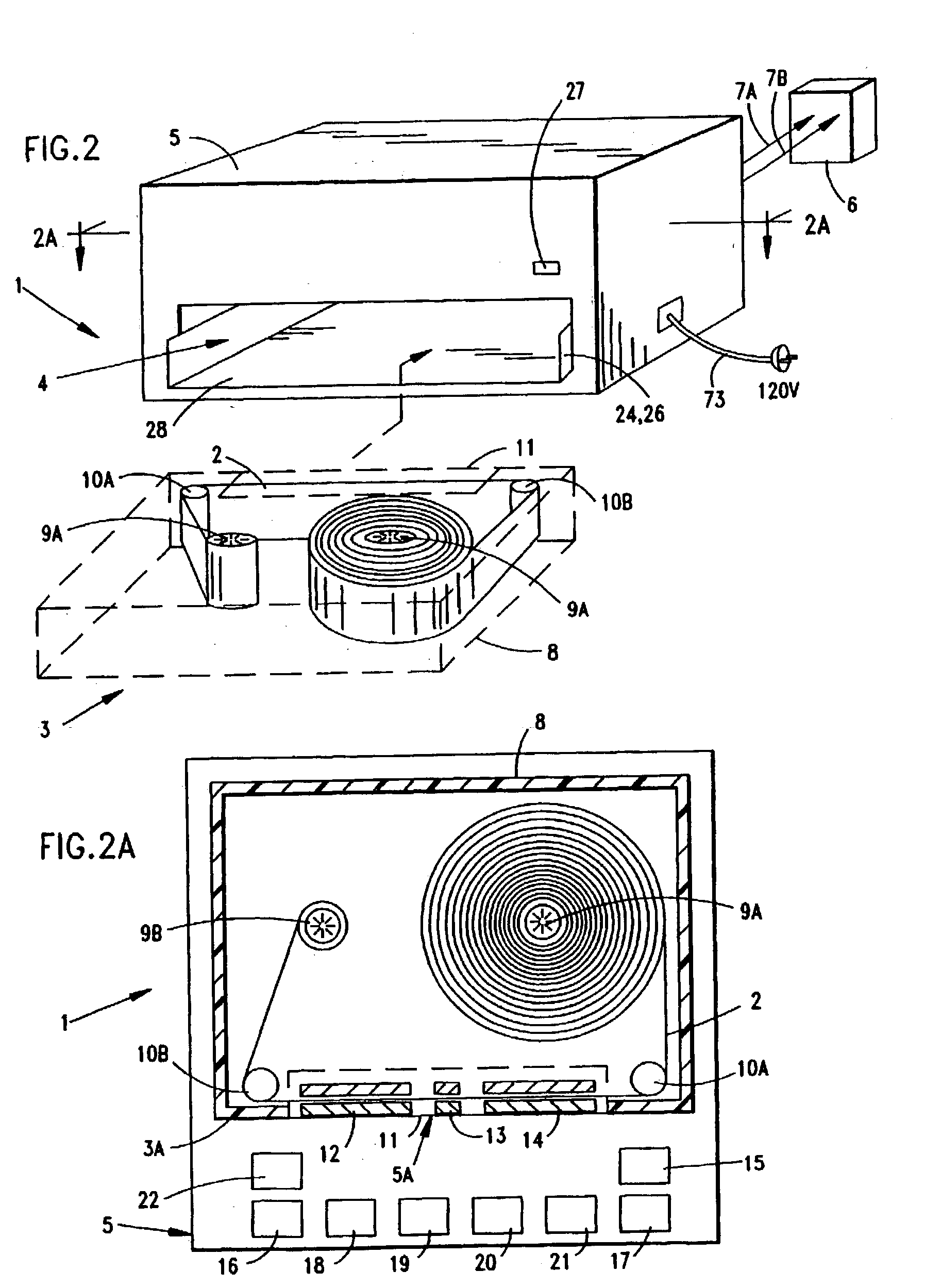
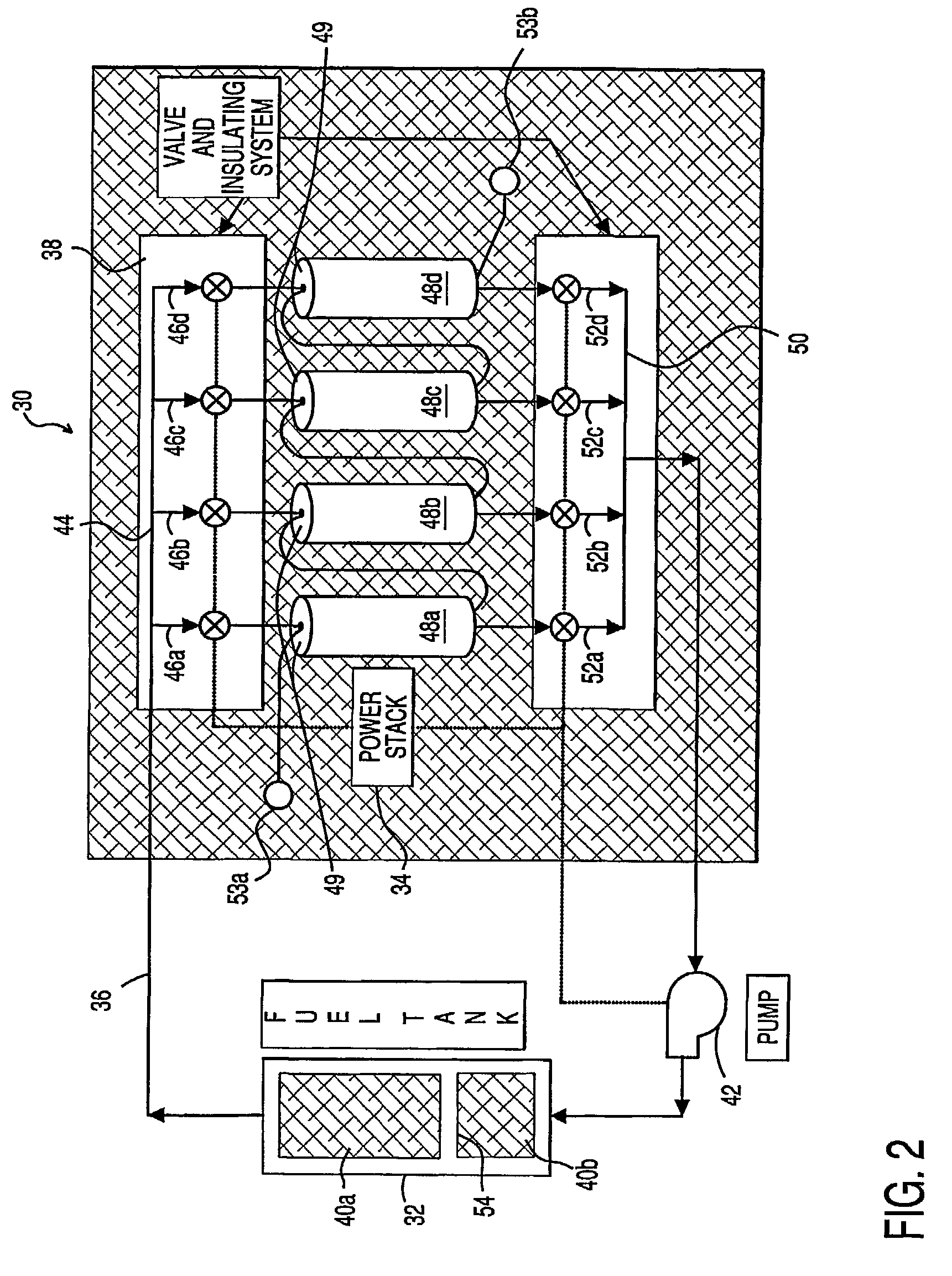
System and method for producing electrical power using metal-air fuel cell battery technology
InactiveUS20040247969A1Fast chargingFuel and primary cellsMoving electrode arrangementsElectricityFuel cells
Improved metal-air fuel cell battery systems having metal-fuel realized in the form of metal-fuel tape cartridges and metal-fuel cards, which can be either manually or automatically inserted within the power generation bay of the system. In order to produce a range of output voltages, the metal-fuel tape has a plurality of electrically-isolated metal-fuel tracks and the metal-fuel cards have a plurality of electrically-isolated metal-fuel strips. An output voltage configuration subsystem is provided for configuring the voltages produced by the individual cells to produce a desired output. A subsystem is provided for detecting oxide formation on the metal-fuel tracks and strips so that only metal-fuel that has been oxidized is reduced during recharging operations. A subsystem is also provided for controlling the flow of oxygen into the power generation head in order to control the power output from the system.
Owner:FARIS SADEG M +3
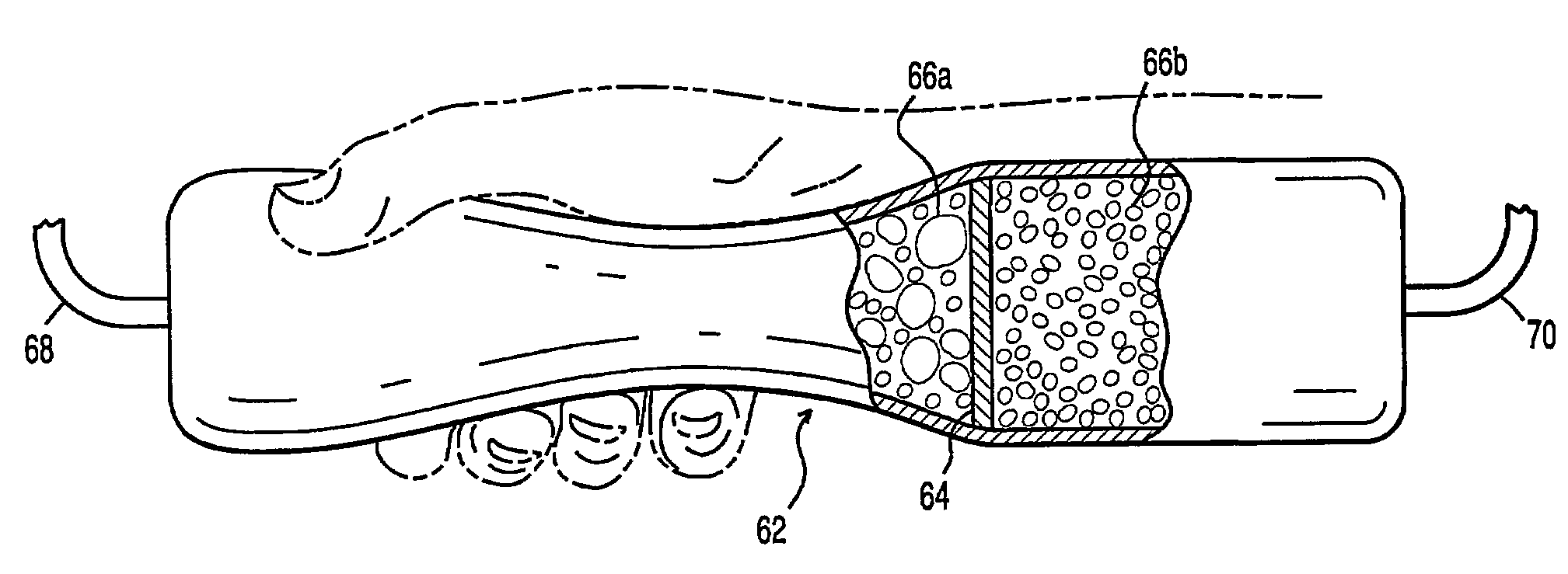
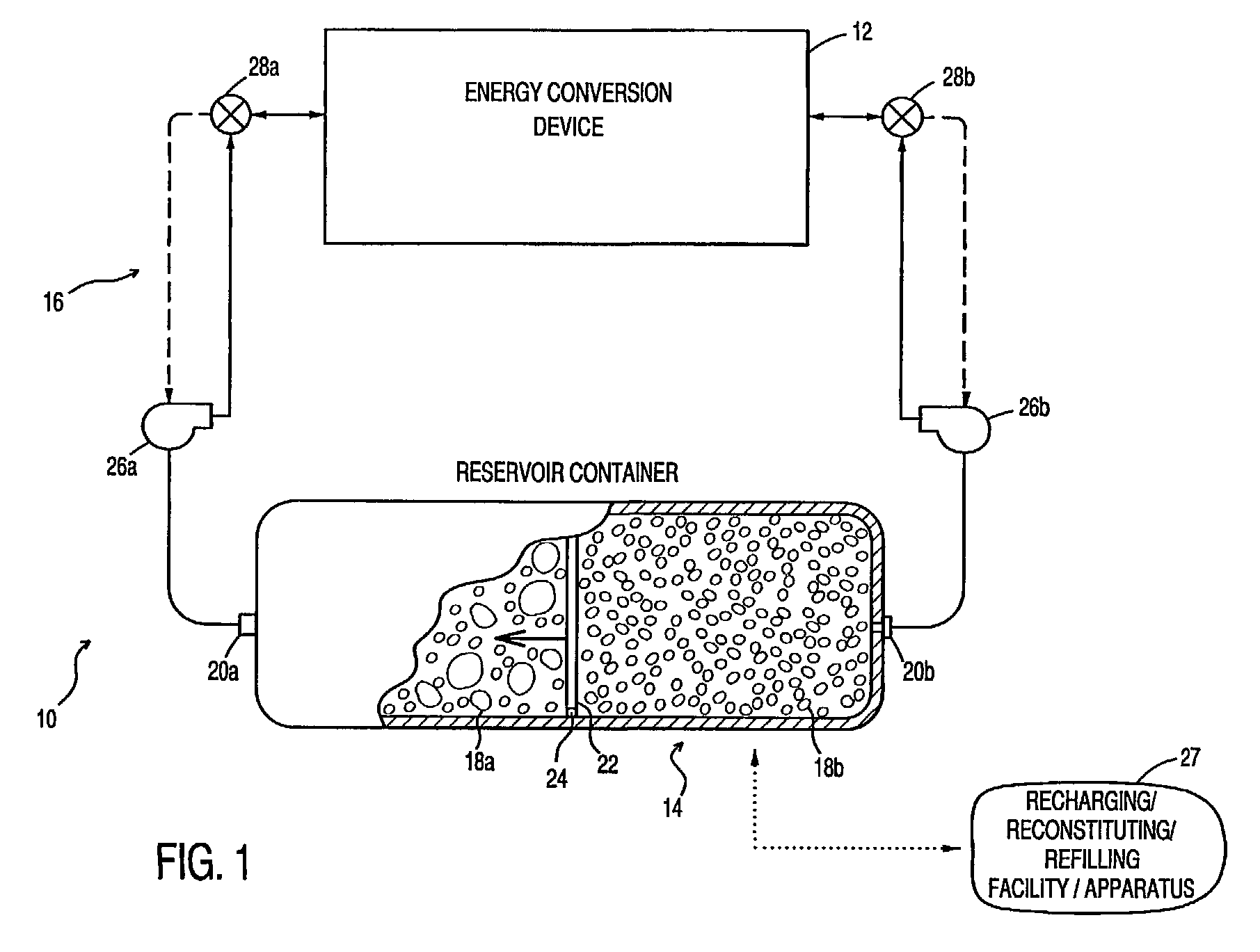
Fuel containment and recycling system
InactiveUS7226676B2Improve economyEliminate short-circuitingFuel and primary cellsSeveral cell simultaneous arrangementsFuel cellsElectric power system
An energy conversion system, comprising: a reservoir container including at least two chambers of inversely variable volume for respectively storing a quantity of fuel and receiving a quantity of exhaust; a means for decreasing the volume of the first chamber while concurrently increasing the volume of the second chamber; at least one energy conversion device; first means for communicating fuel between the at least one energy conversion device and a first of the chambers in the reservoir container; and second means for communicating exhaust between the at least one energy conversion device and a second of the chambers in the reservoir container. The reservoir container may be transported to a recharging / refilling station or recharged in-situ. A particular application for metal-air fuel cell power systems is shown and described.
Owner:REVEO
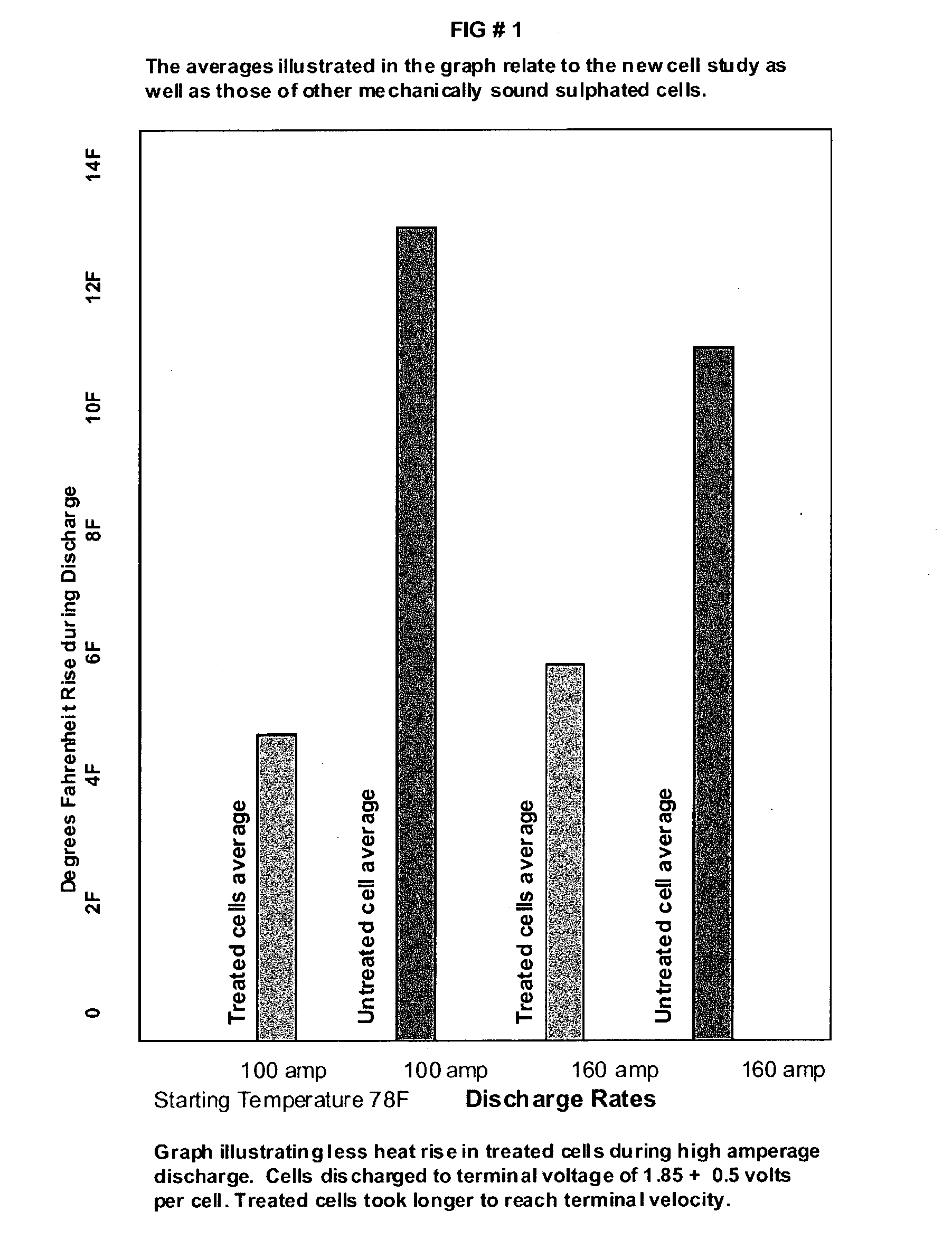
Battery life extender additives
InactiveUS20040018427A1Increase battery capacityAlkaline accumulatorsLead-acid accumulatorsEngineeringBoric acid
The present invention relates to chemicals or a combination of chemicals for optimizing the function of a new and already in service lead-acid battery over a long period of time, reducing corrosion on the positive plate, restoring lost capacity due to the accumulation of non-conductive lead sulphate crystals on plates, reducing charging time, and for starting batteries increasing cold crank rating. The chemicals, Sodium Hydroxide and Sodium Tetraborate Decahydrate, can be used separately or in combination either in their solid form or dissolved in de-ionized water. Other chemicals, Sodium Chloride and Boric Acid can be used in combination either in their solid form or dissolved in de-ionized water. Those chemicals can be effective in all types of lead-acid batteries, including flooded (wet), gel and absorbed glass mat (AGM) lead-acid VRLA (Valve Regulated Lead Acid) batteries used as starting, storage, power, standby (back-up and Uninterrupted Power System), marine, industrial batteries.
Owner:MONCONDUIT ROBERT A
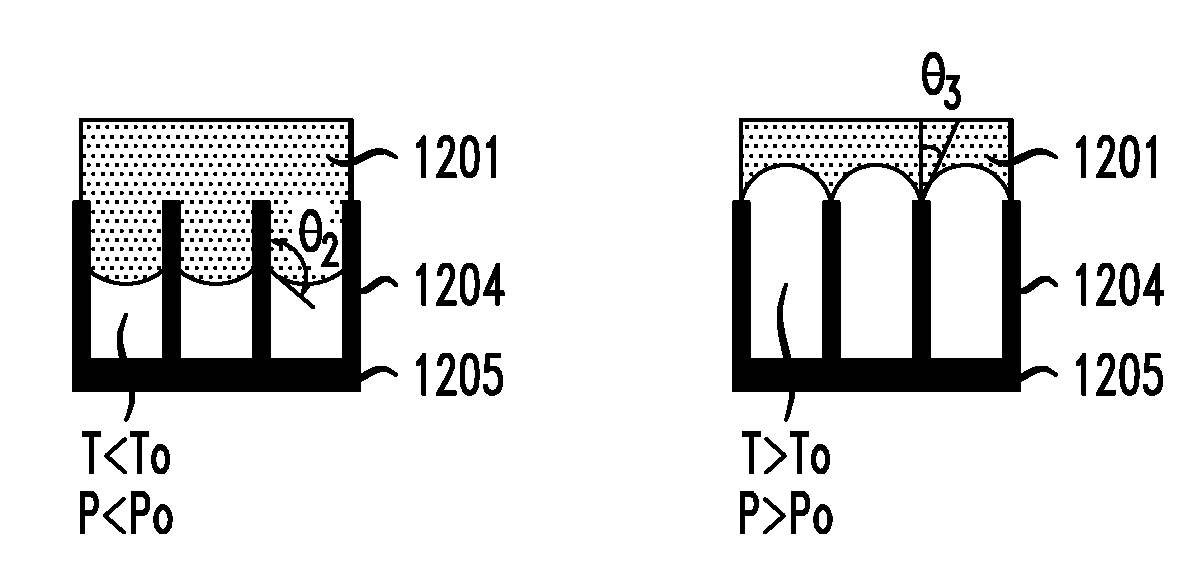
Reversibly-activated nanostructured battery
InactiveUS20050208370A1Material nanotechnologyDeferred-action cellsNanostructureBiomedical engineering
A battery having a nanostructured battery electrode is disclosed wherein it is possible to reverse the contact of the electrolyte with the battery electrode and, thus, to return a battery to a reserve state after it has been used to generate current. In order to achieve this reversibility, the nanostructures on the battery electrode comprise a plurality of closed cells and the pressure within the enclosed cells is varied. In a first embodiment, the pressure is varied by varying the temperature of a fluid within the cells by, for example, applying a voltage to electrodes disposed within said cells. In a second illustrative embodiment, once the battery has been fully discharged, the battery is recharged and then the electrolyte fluid is expelled from the cells in a way such that it is no longer in contact with the battery electrode.
Owner:ALCATEL-LUCENT USA INC
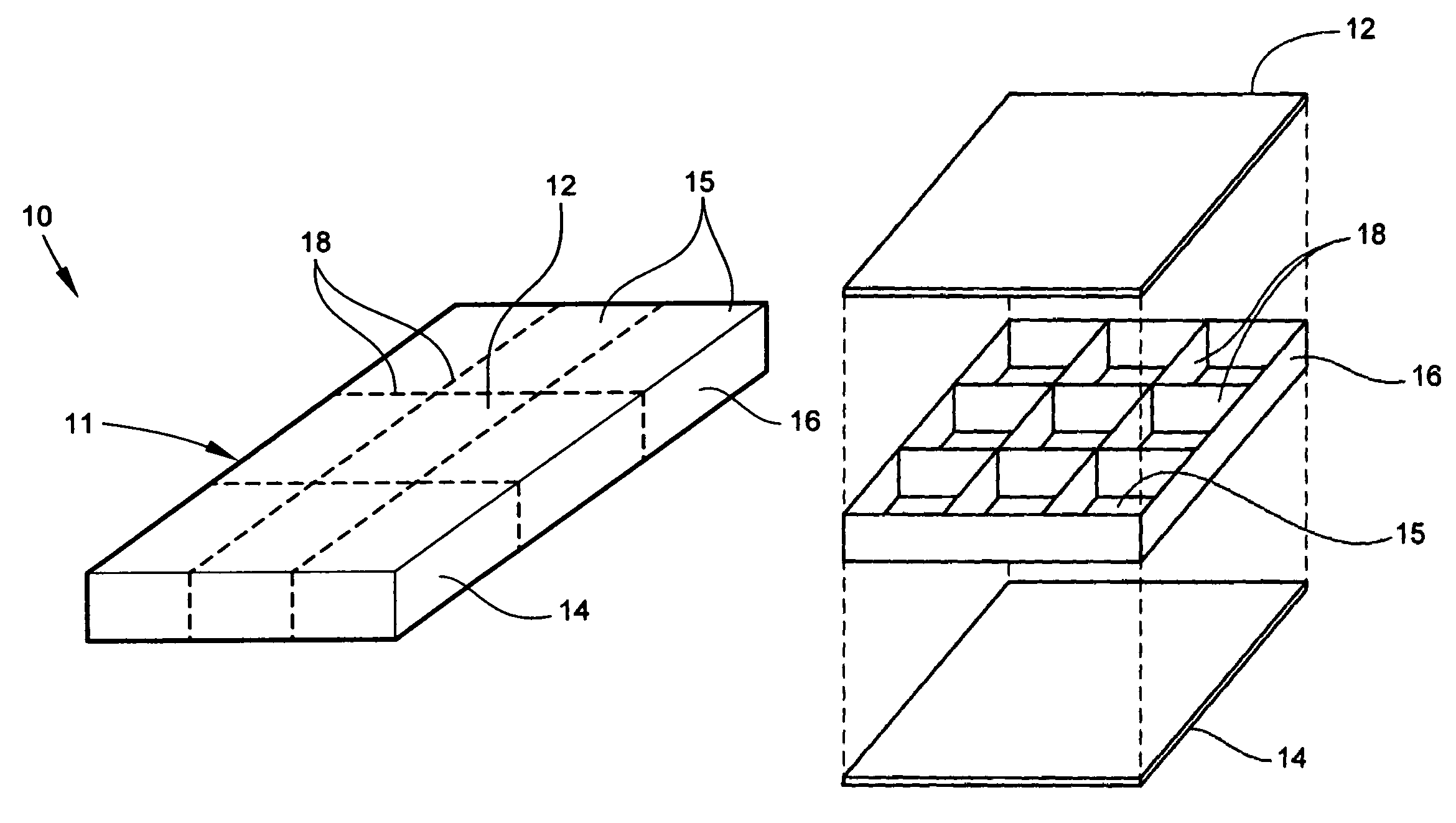
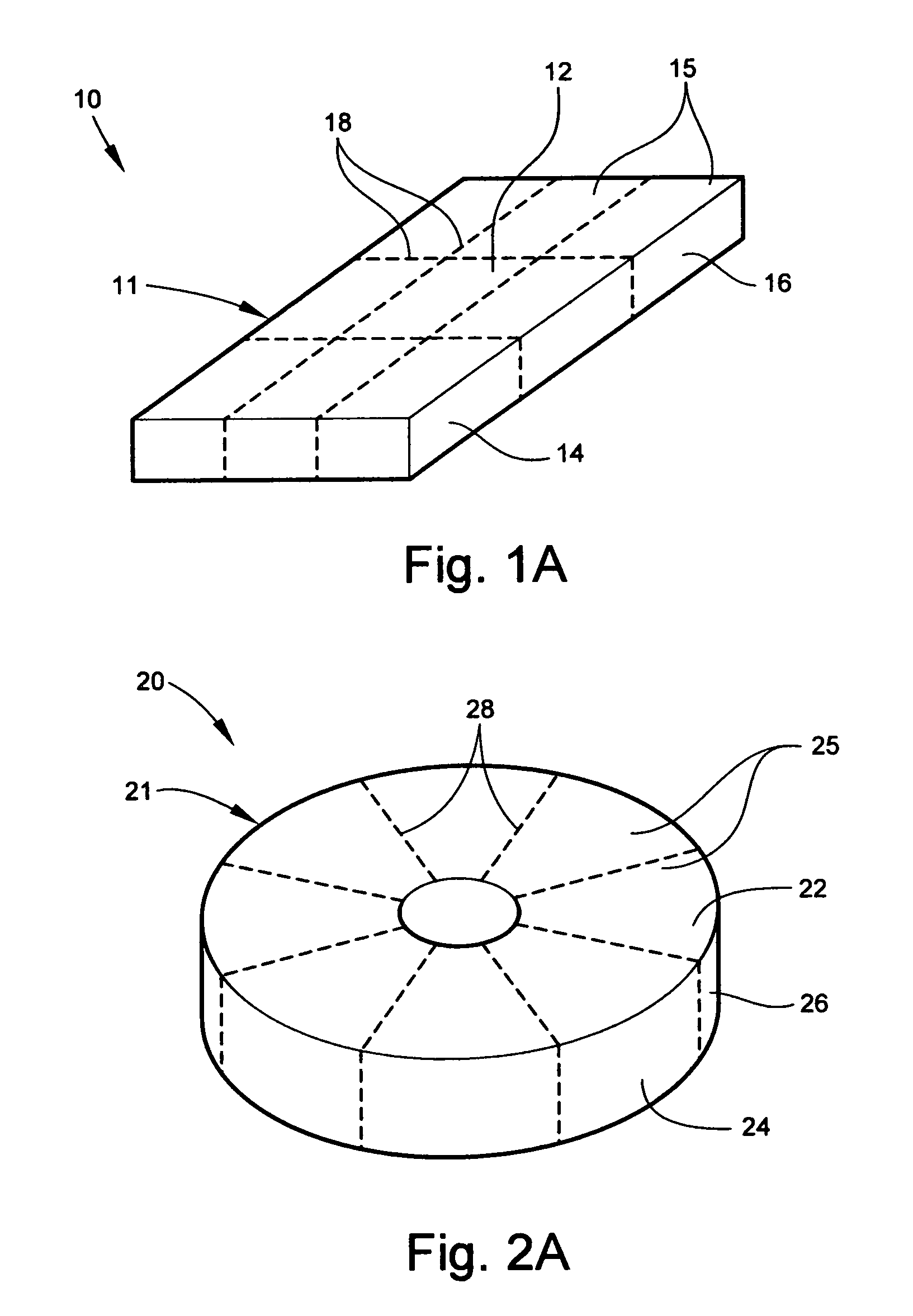
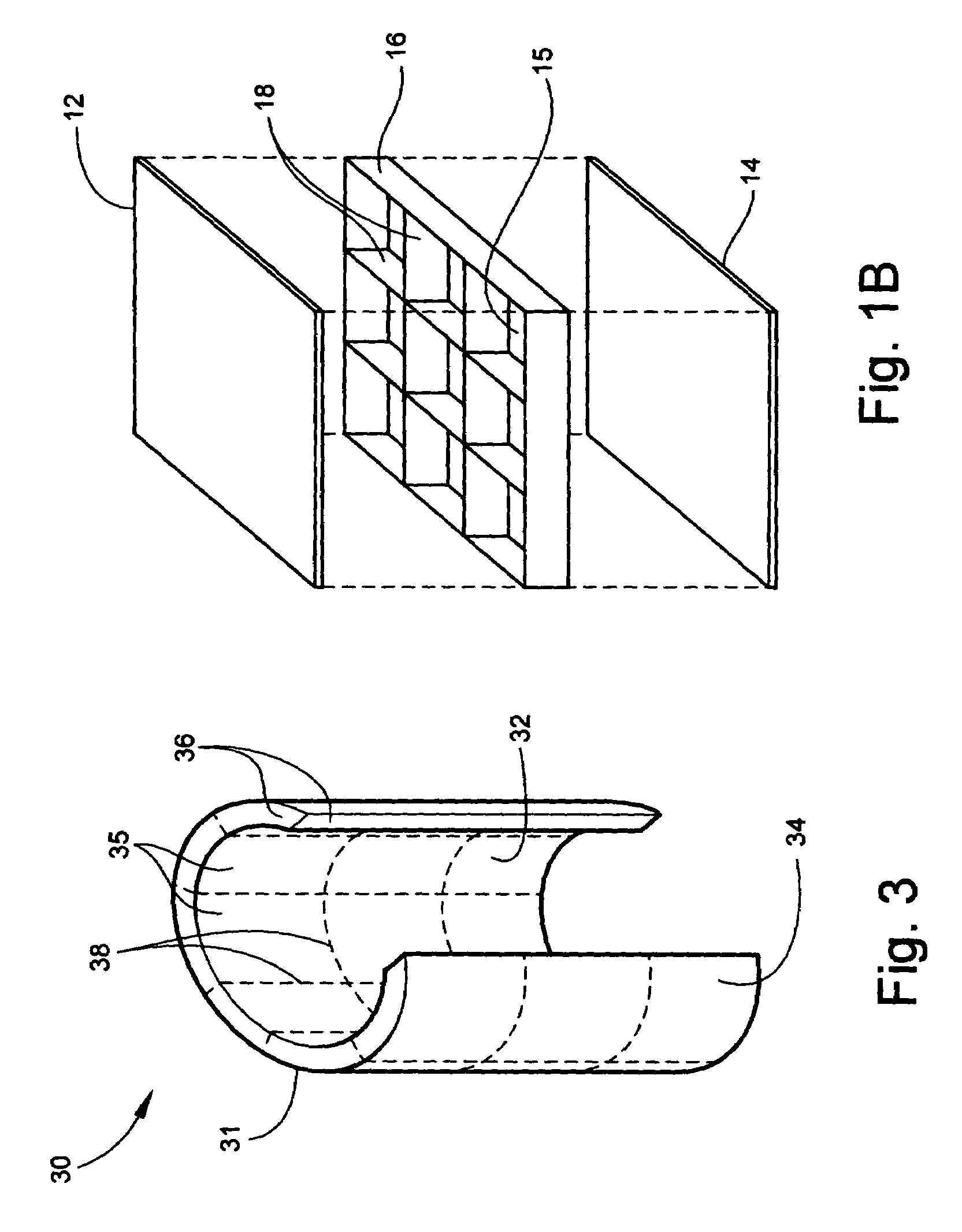
Conformable battery
InactiveUS7479344B1Increase surface areaImprove heat transfer performanceElectrolyte moving arrangementsMoving electrode arrangementsPower modeInternal pressure
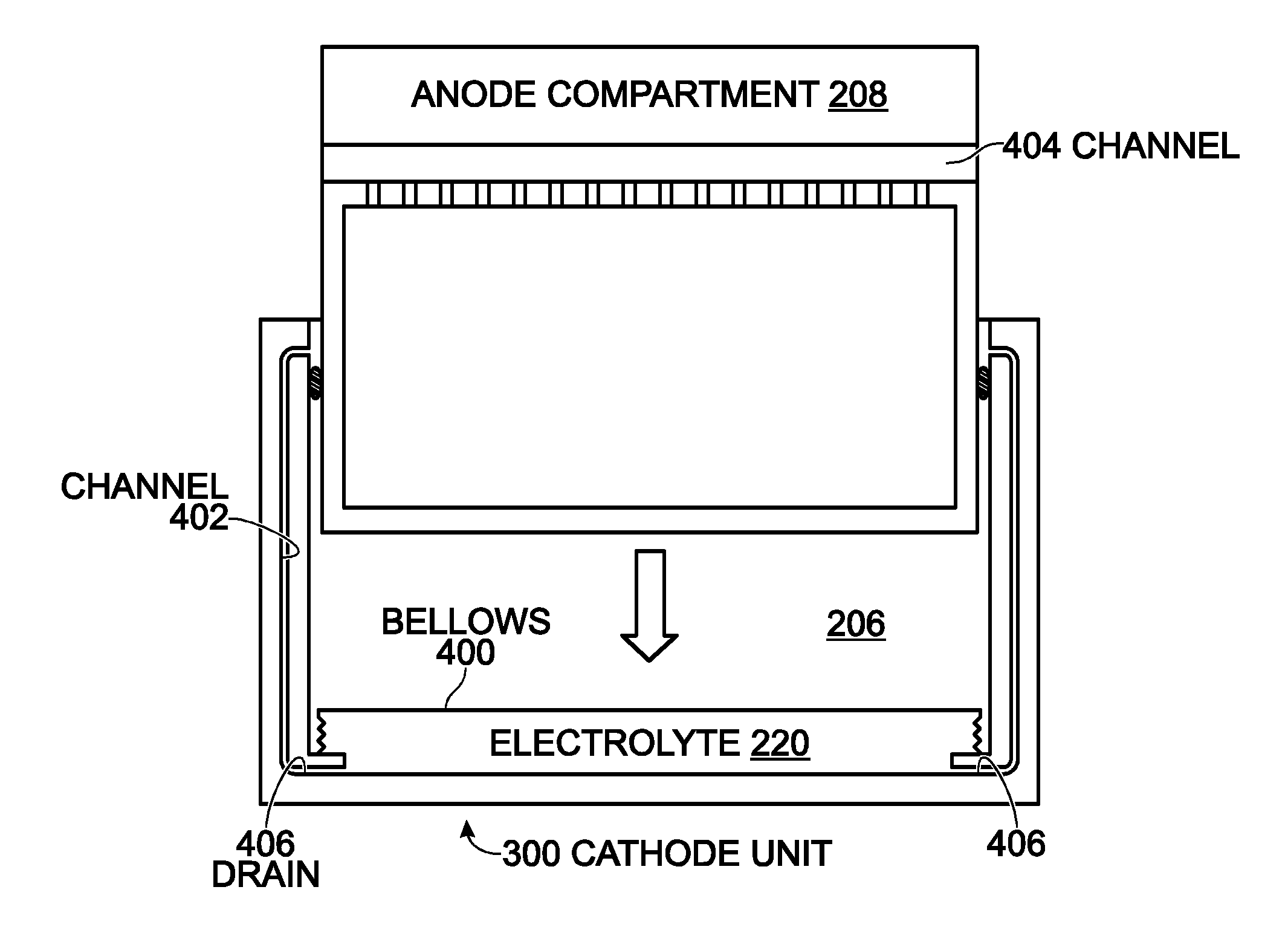
A conformable battery wherein the outer casing has an upper face plate, a lower face plate, and at least one perimetric wall, and an interior of the battery comprising a grid of walls extending from the upper face plate to the lower face plate and connecting to the at least one perimetric wall, thereby dividing the interior of the battery into at least two compartments and increasing the battery's structural stiffness and ability to sustain increased internal pressure. Each compartment contains an electrochemically active plate stack, and a network of electrical conductors provides electrical connection between the plate stacks in each of the compartment. The battery may further comprise a reservoir containing an acid additive which, when released into each of the compartments, shifts the battery from a low power mode into a high power mode.
Owner:MCDERMOTT PATRICK P
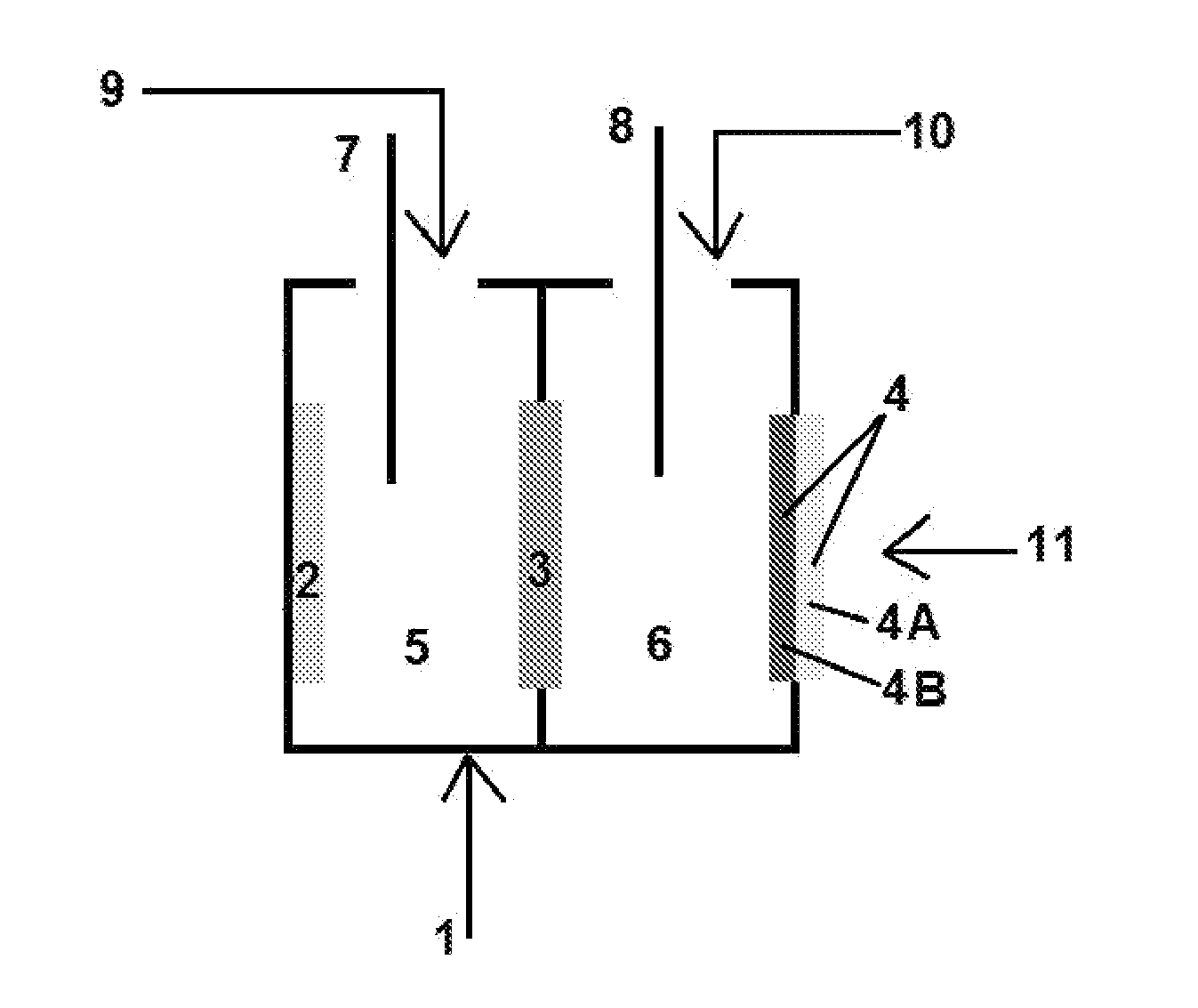
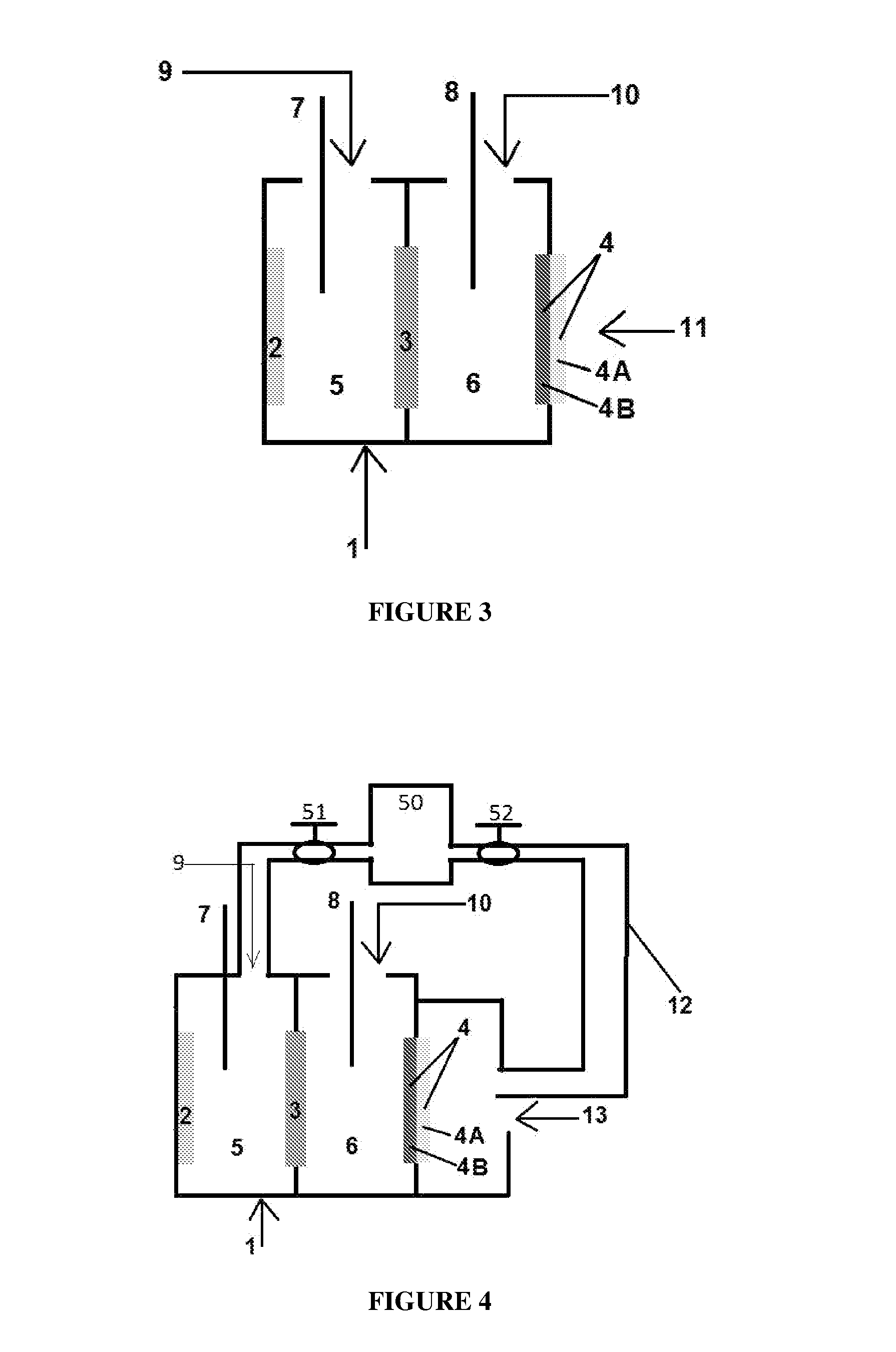
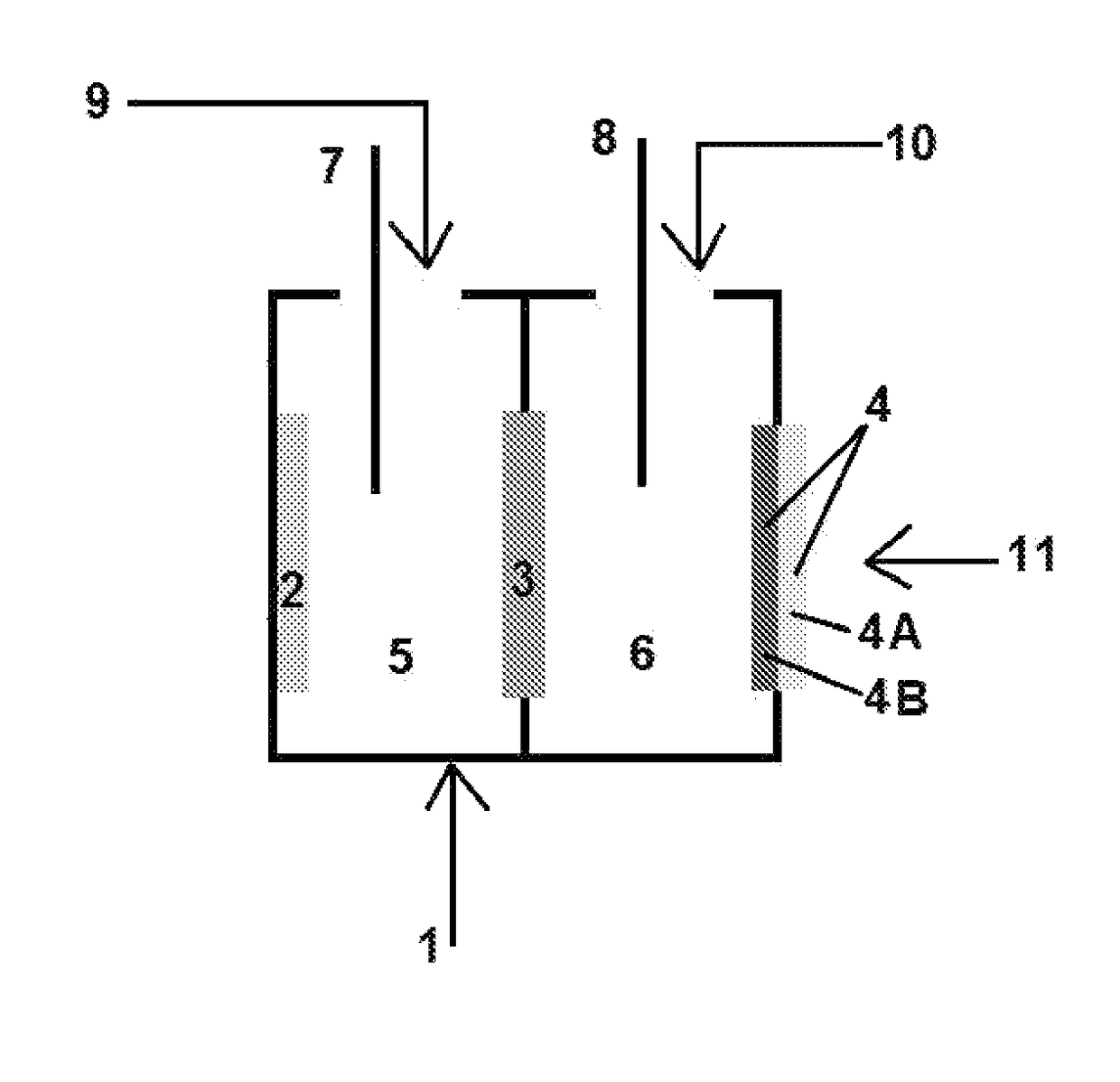
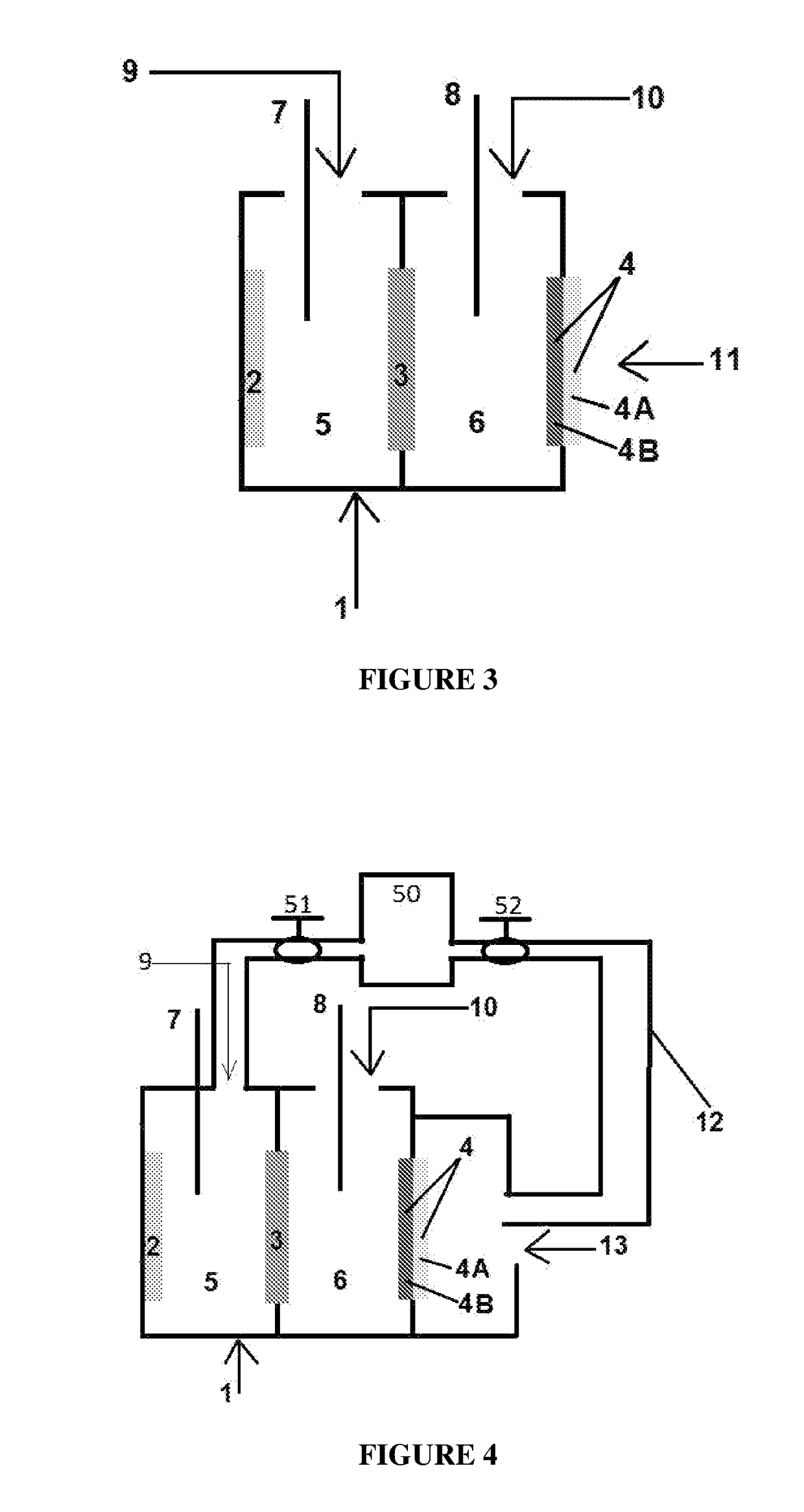
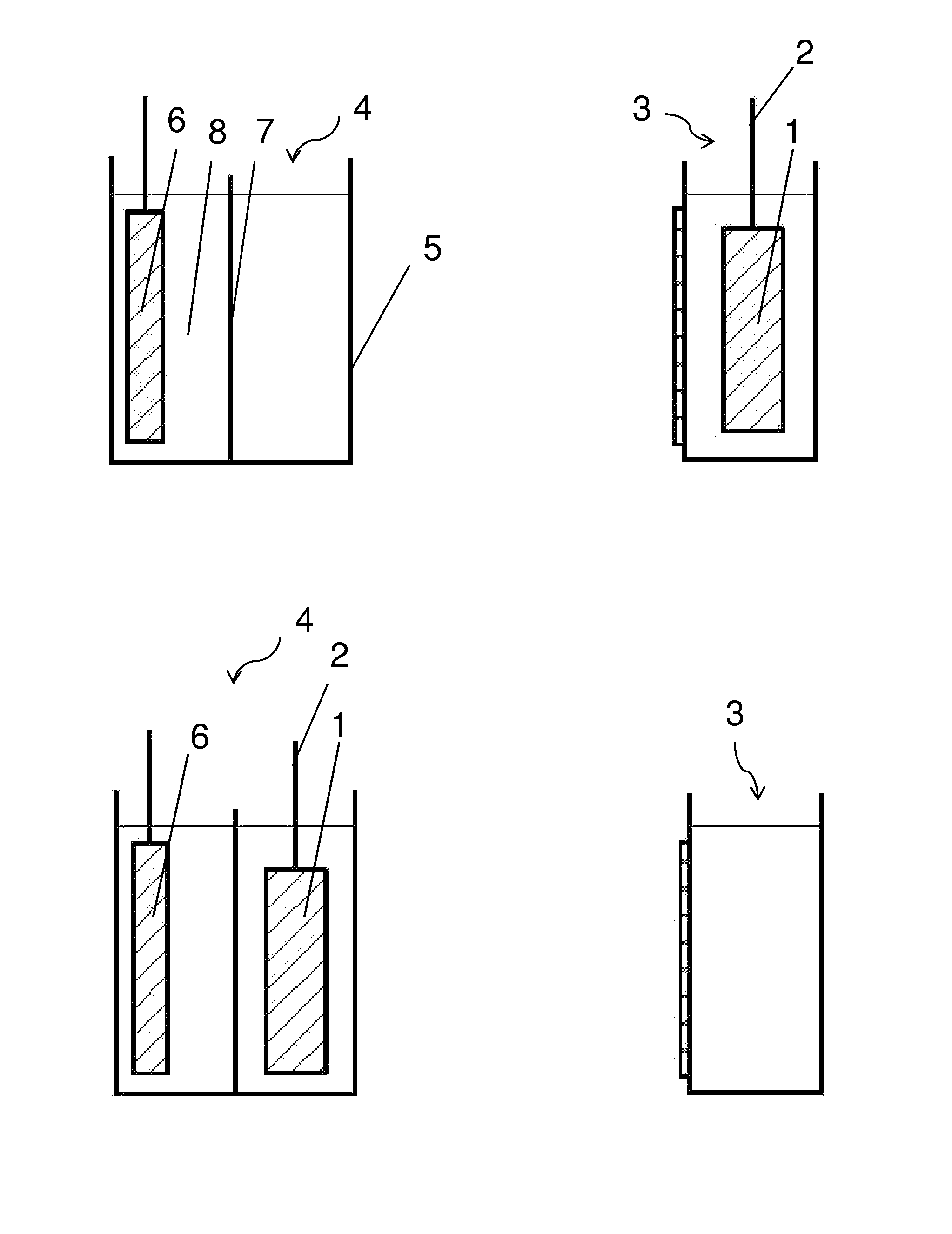
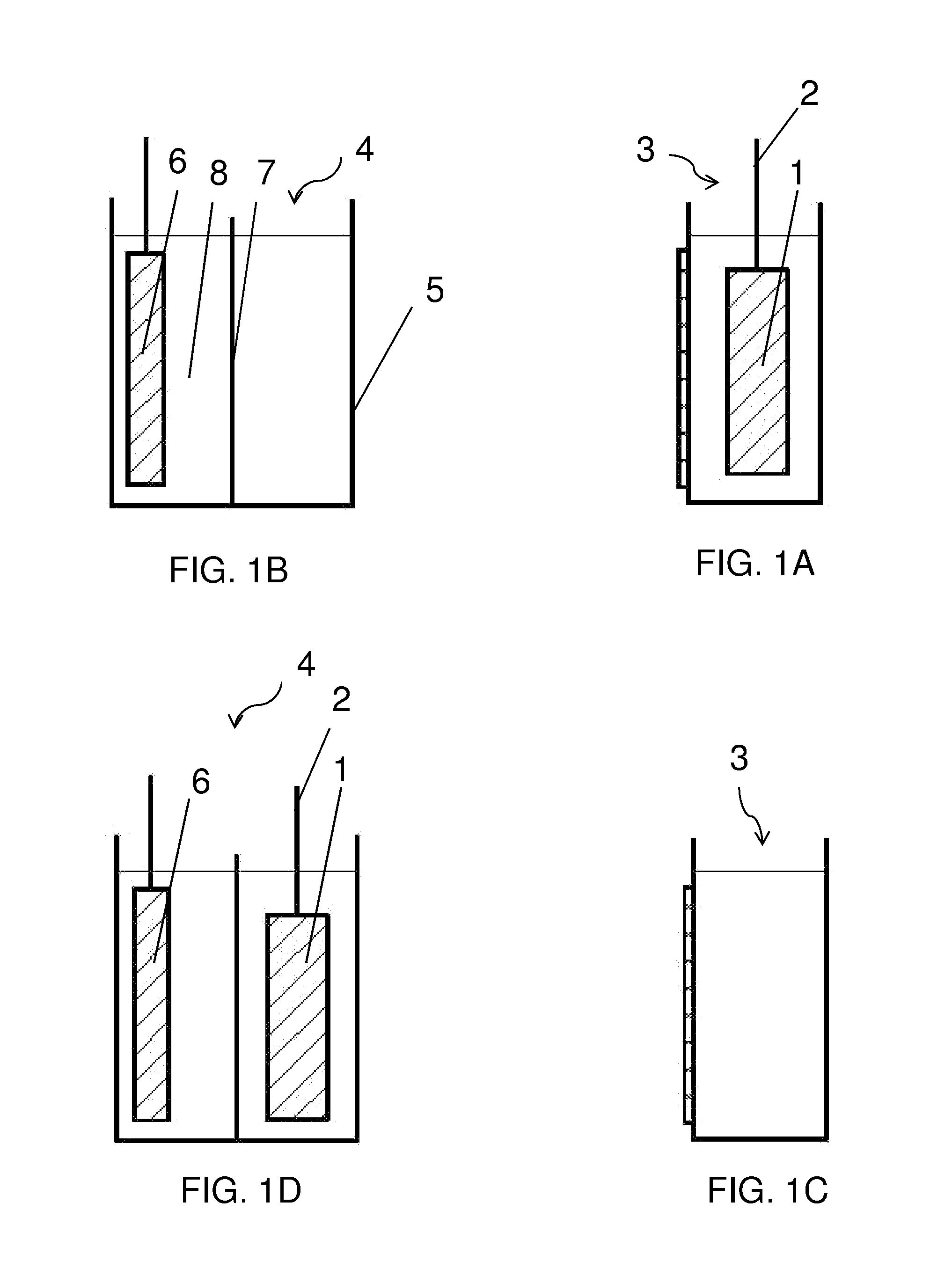
Electrolyte regeneration
ActiveUS20160149231A1Reduce concentrationImprove concentrationFuel and primary cellsPhotography auxillary processesElectrolysisPhysical chemistry
This invention is directed to electrolysis-based devices and methods for recycling of electrolyte solutions. Specifically, the invention is related to regeneration of spent electrolyte solutions comprising metal ions such as electrolyte solutions used in metal / air batteries.
Owner:PHINERGY
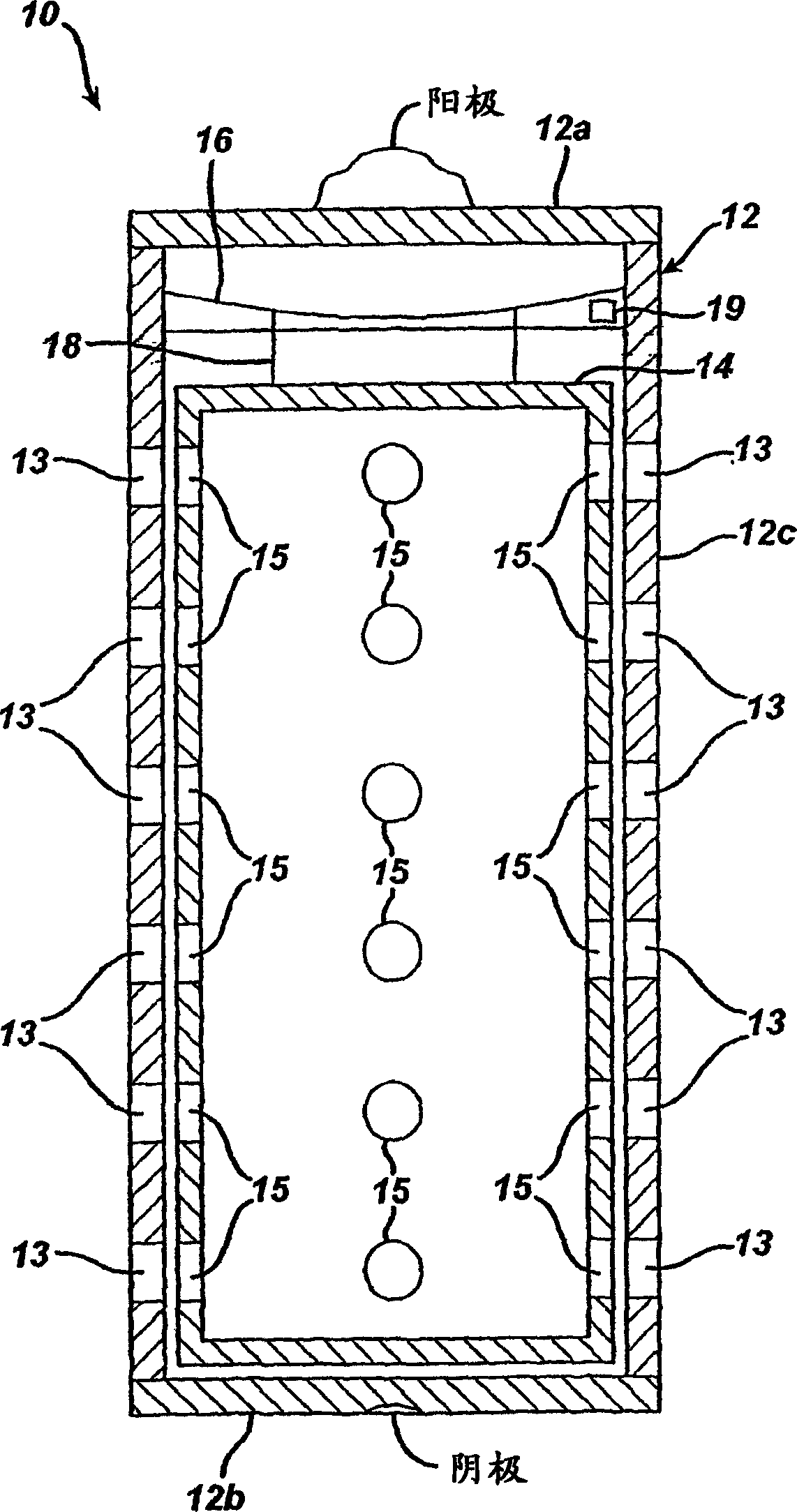
Metal-air battery
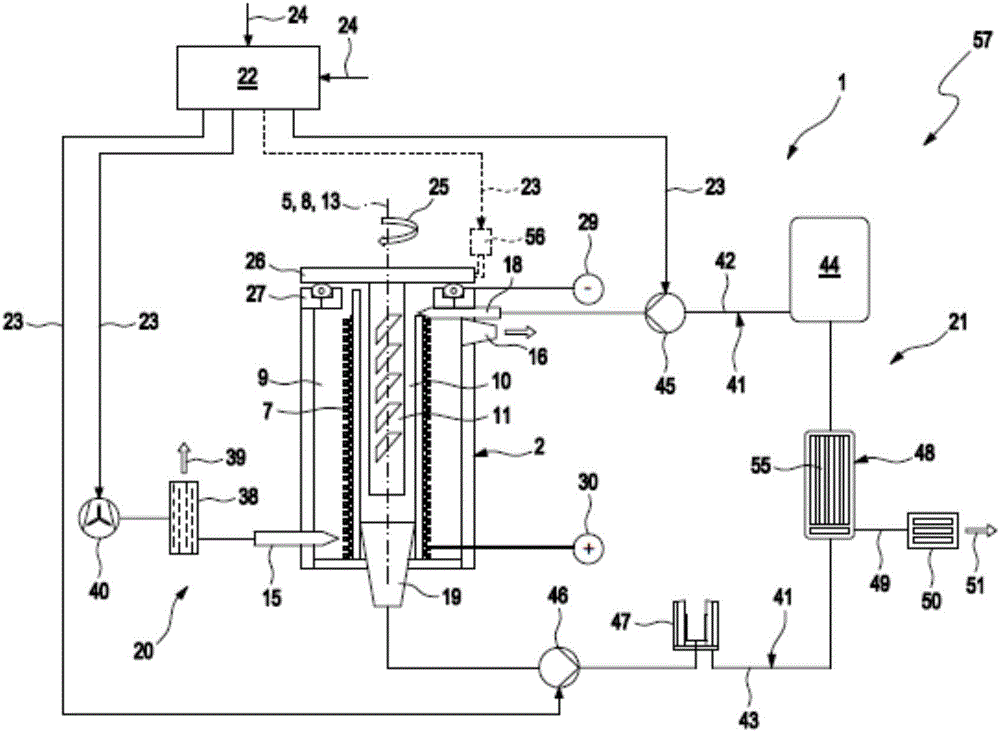
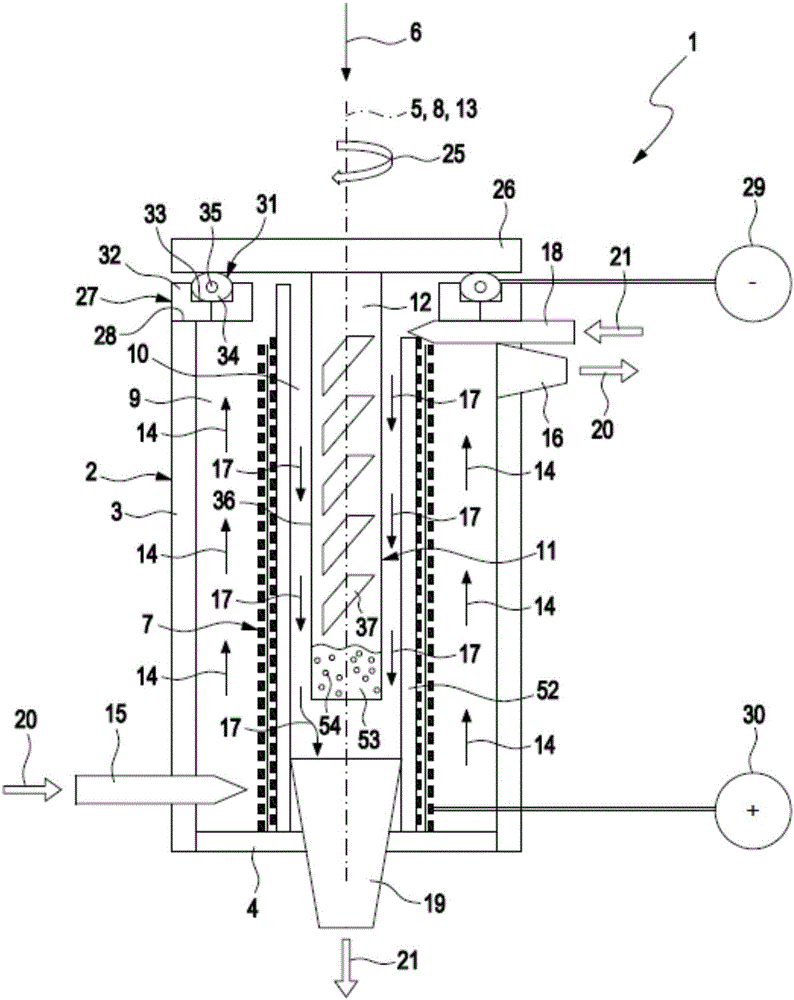
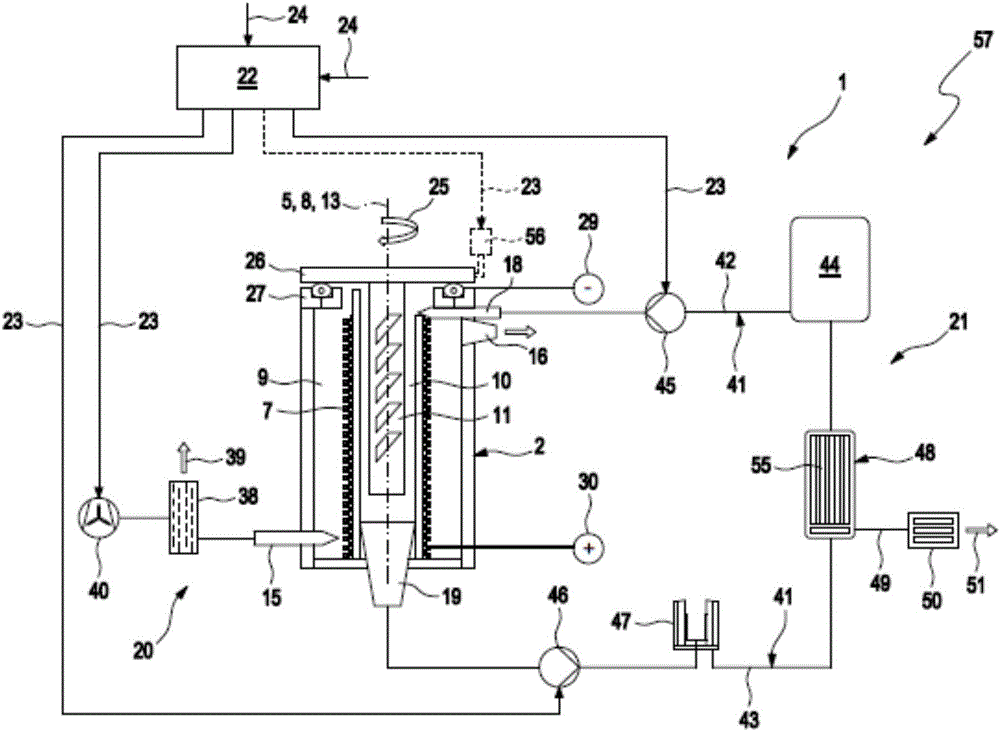
ActiveCN106463664AExtended service lifeEasy to change and relatively fastFuel and primary cellsFuel and secondary cellsEngineeringMetal
The invention relates to a metal-air battery (1), comprising a housing (2), a hollow cylindrical cathode (7), which is arranged between an air chamber (9) and an electrolyte chamber (10) in the housing (2), a metal anode (11), which is arranged in the electrolyte chamber (10), an air path (14) leading through the housing (2), which air path leads from an air inlet (15) of the housing (2) to an air outlet (16) of the housing (2), an air supply device (20) for producing an air flow, which follows the air path (14) and impinges on the cathode (7), an electrolyte path (17) leading through the housing (2), which electrolyte path leads from an electrolyte inlet (18) of the housing (2), said electrolyte inlet being fluidically connected to the electrolyte chamber (10), to an electrolyte outlet (19) of the housing (2), said electrolyte outlet being fluidically connected to the electrolyte chamber (10), and an electrolyte supply device (21) for producing an electrolyte flow, which follows the electrolyte path (17) and impinges on the anode (11) and the cathode (7).
Owner:MAHLE INT GMBH
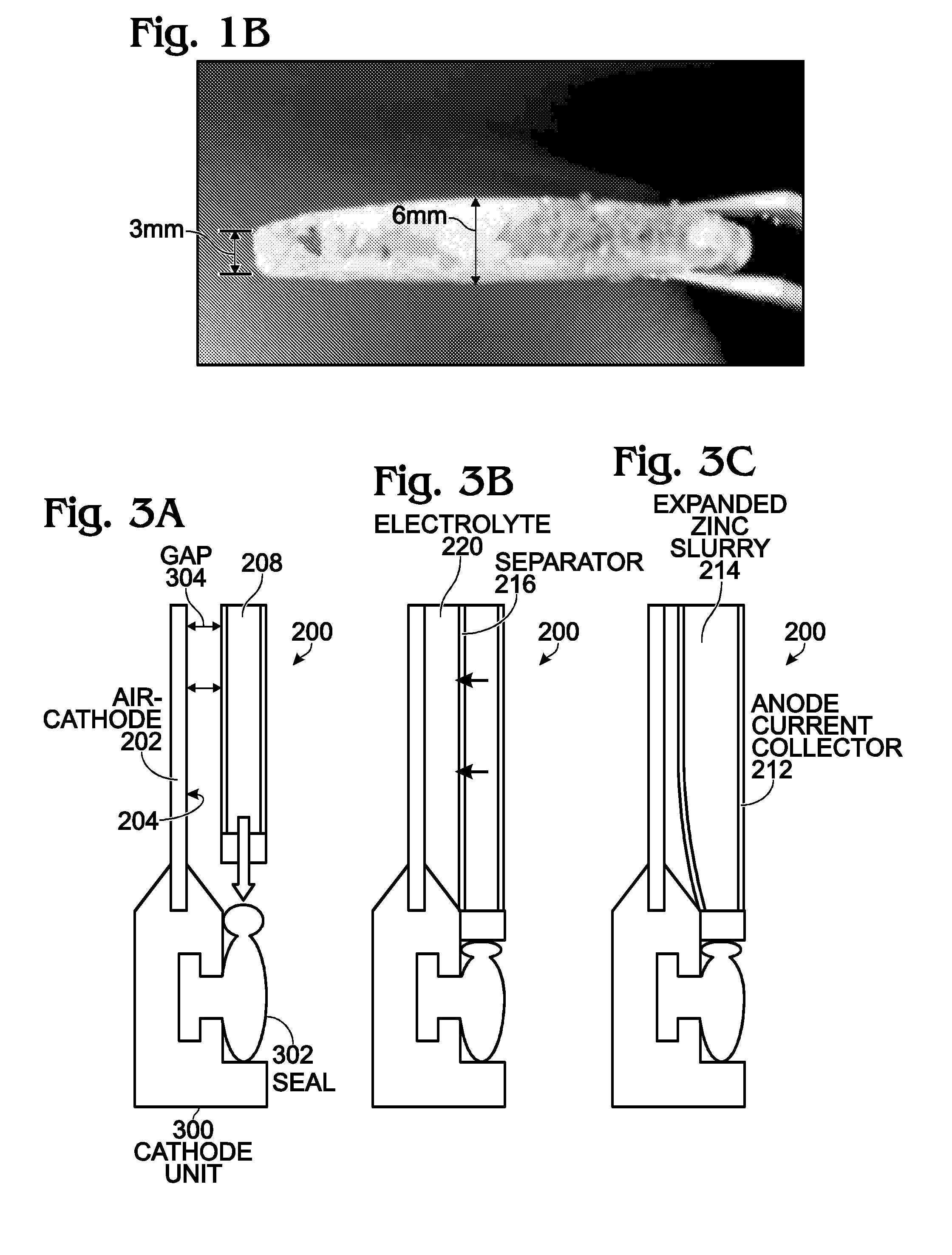
Air Cathode Battery Using Zinc Slurry Anode with Carbon Additive
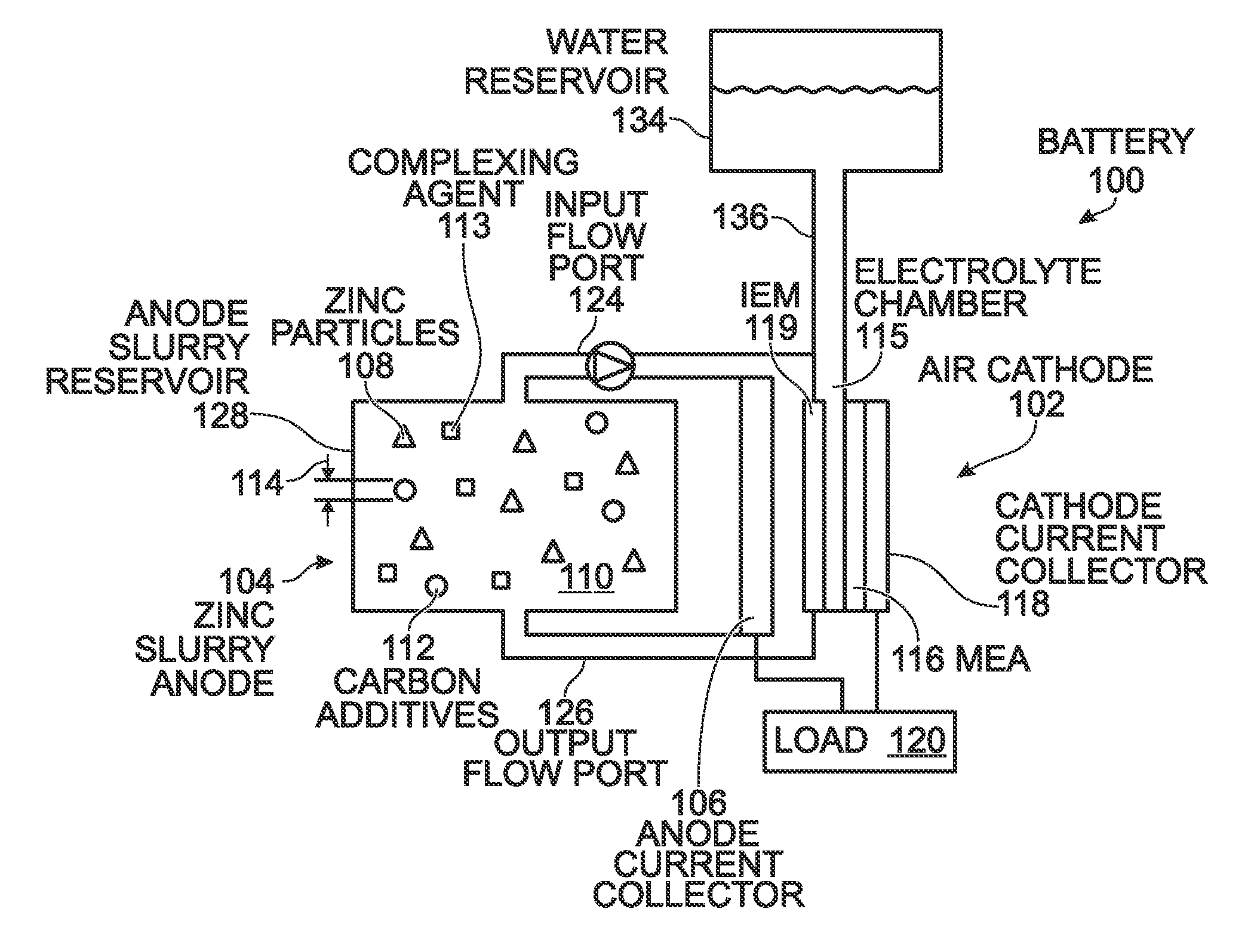
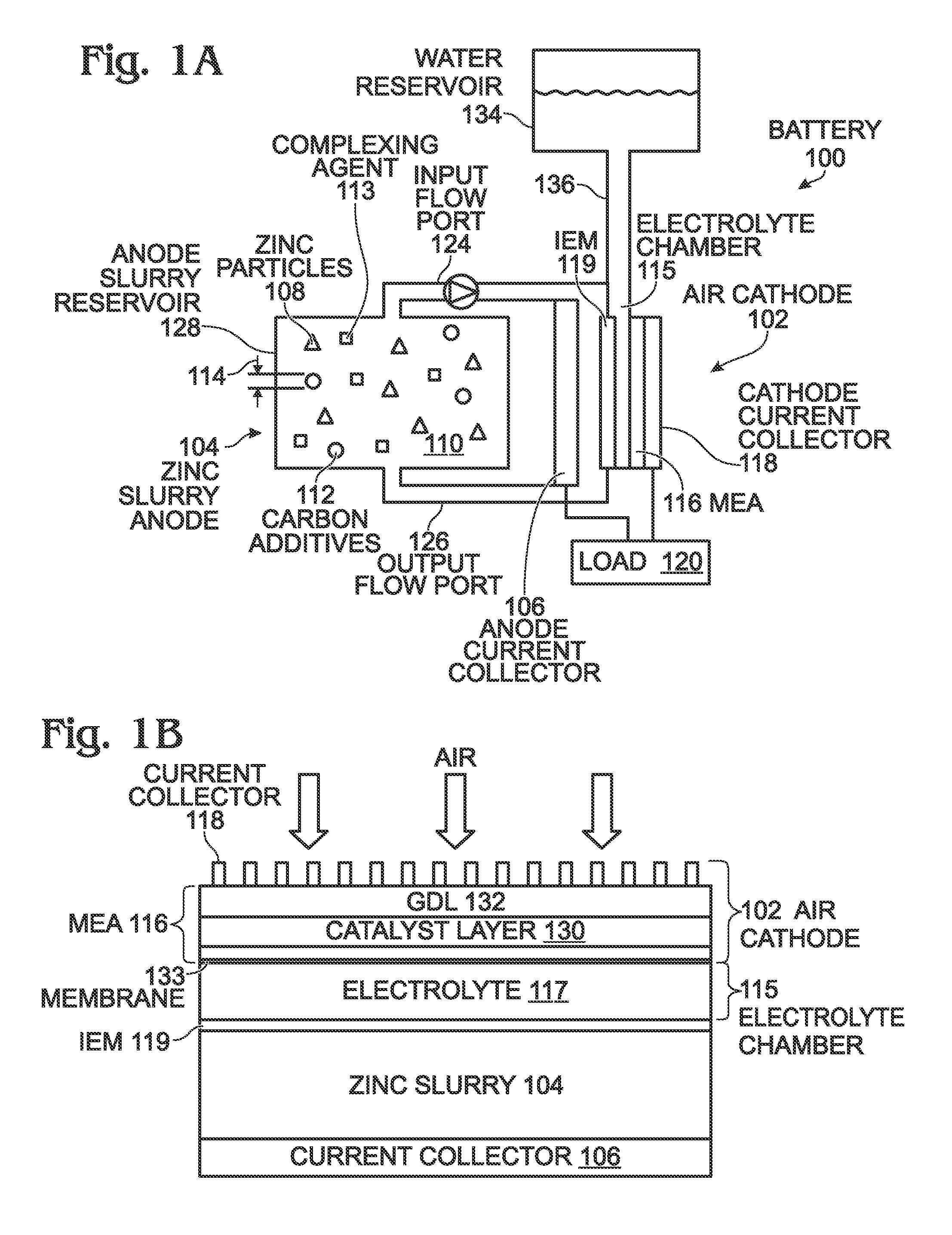
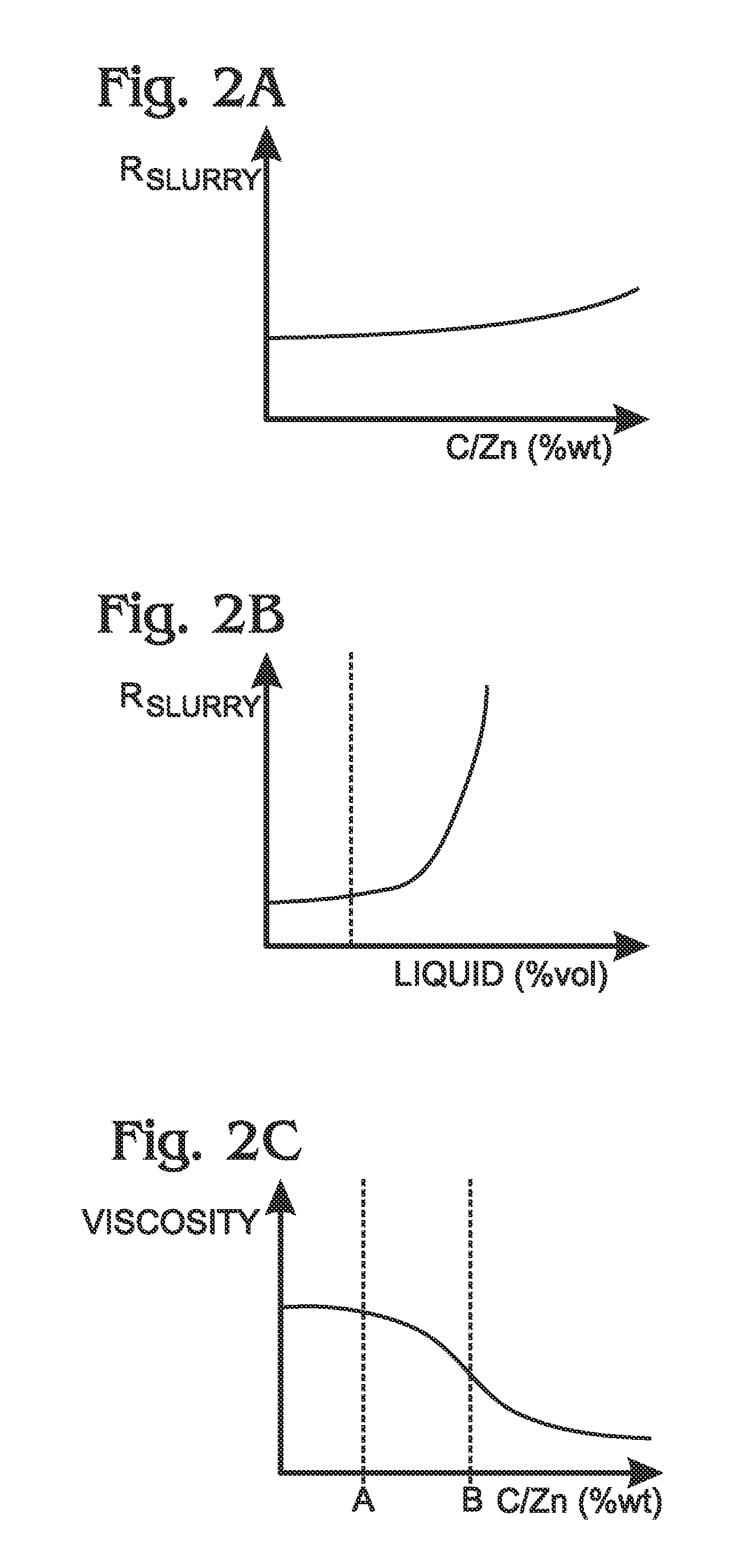
ActiveUS20140370401A1Scalable capacityLow costFuel and primary cellsFuel and secondary cellsFiberCarbon Additive
An air cathode battery is provided that uses a zinc slurry anode with carbon additives. The battery is made from an air cathode and a zinc slurry anode. The zinc slurry anode includes zinc particles, an alkaline electrolyte, with a complexing agent and carbon additives in the alkaline electrolyte. A water permeable ion-exchange membrane and electrolyte chamber separate the zinc slurry from the air cathode. The carbon additives may, for example, be graphite, carbon fiber, carbon black, or carbon nanoparticles. The proportion of carbon additives to zinc is in the range of 2.5 to 10% by weight. The proportion of alkaline electrolyte in the zinc slurry is in the range of 50 to 80% by volume.
Owner:SHARP KK
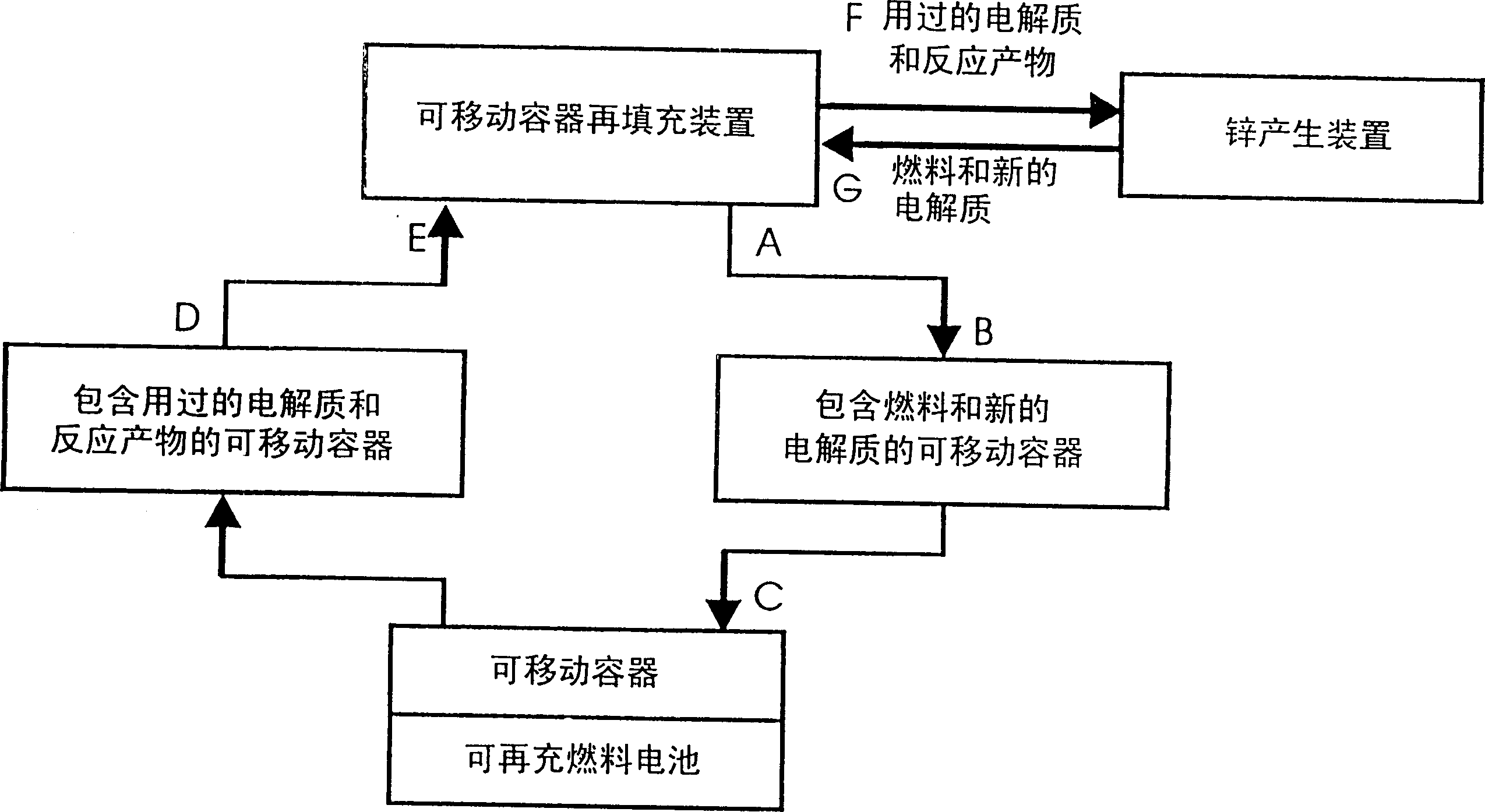
Recyclable fuel distribution, storage, delivery and supply system
InactiveCN1524312AFuel and primary cellsFinal product manufactureChemical reactionReversible reaction
A recyclable fuel distribution system comprising a hierarchy of operatively linked (with fuel supply and reaction product collection elements) fluid fuel transport vehicles, fluid fuel storage tanks, fuel supply reservoirs (optional depending on specific use), and fluid fuel usage devices operatively linked to the storage tanks or supply reservoirs, as applicable. The fuel, during use, undergoes a reversible chemical reaction, whereby collected reaction product is reversible to the original fuel. At least one and preferably all of the vehicles, storage tanks and reservoirs contain a storage volume with separated chambers, adapted to inversely change relative to fuel being supplied and reaction product being collected and stored. As a result, a single volume during transport, storage, and use, provides the dual function of fuel supply and collection of reversible reaction product, with concomitant nearly halving of transport, storage and use volumes and costs. Electrical production with a zinc fuel which reversibly forms a zinc oxide reaction product is a particularly suitable fuel for the present system for use in large scale fuel cell applications. Applications range from large scale megawatt power levels for industrial levels to tens of kilowatts for homes and transportation systems down to several watts for portable electrical and electronic appliances and devices.
Owner:REVEO
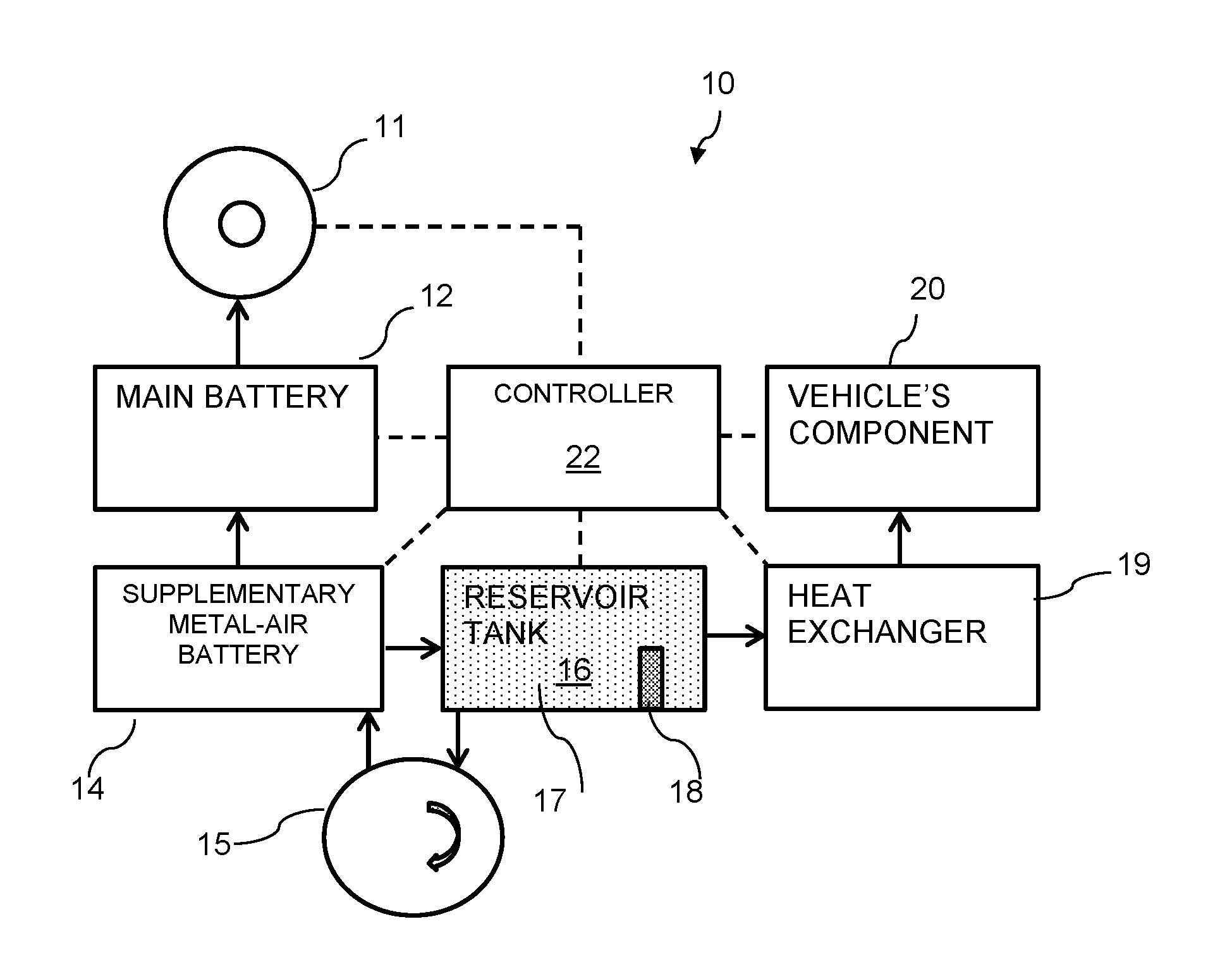
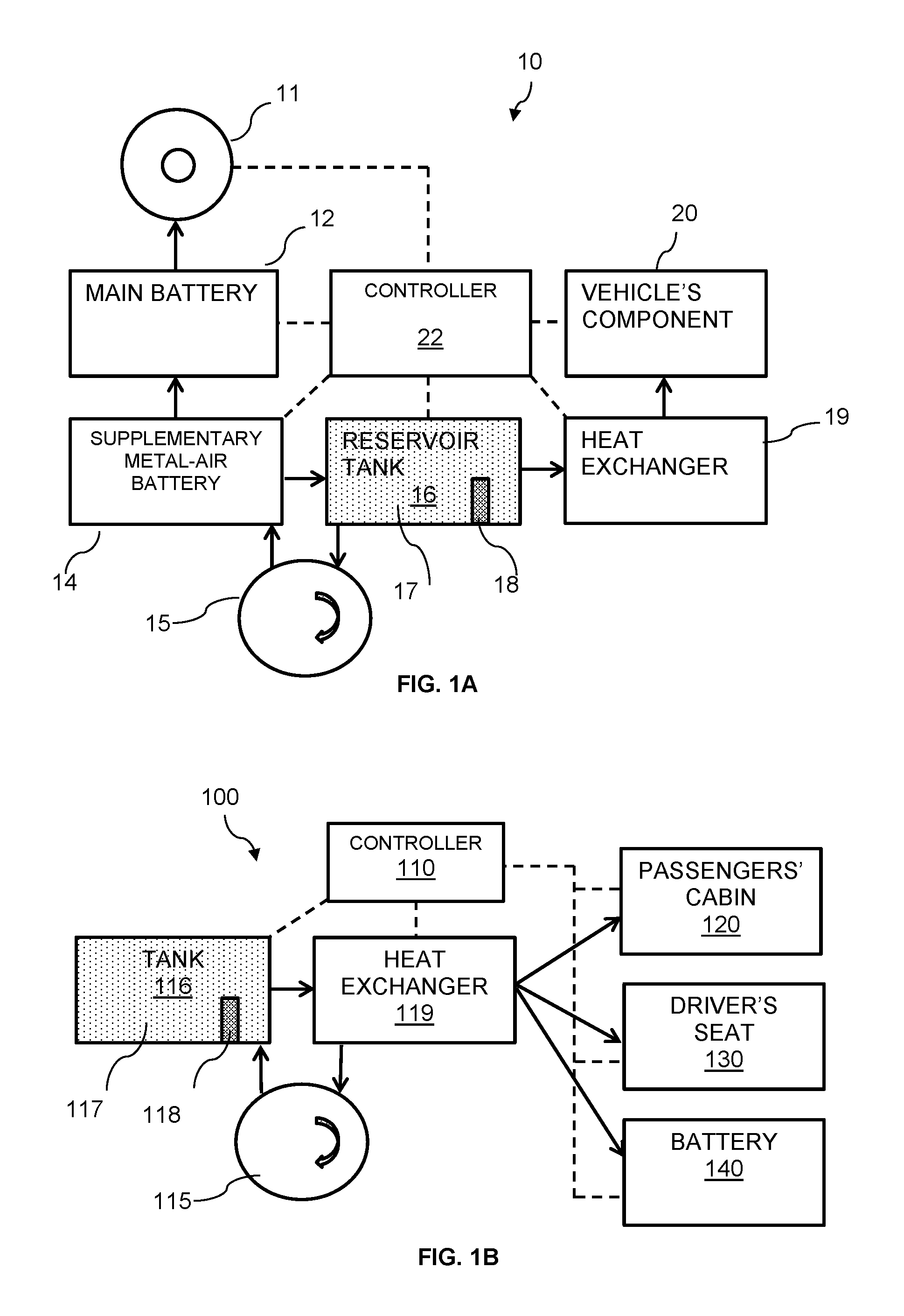
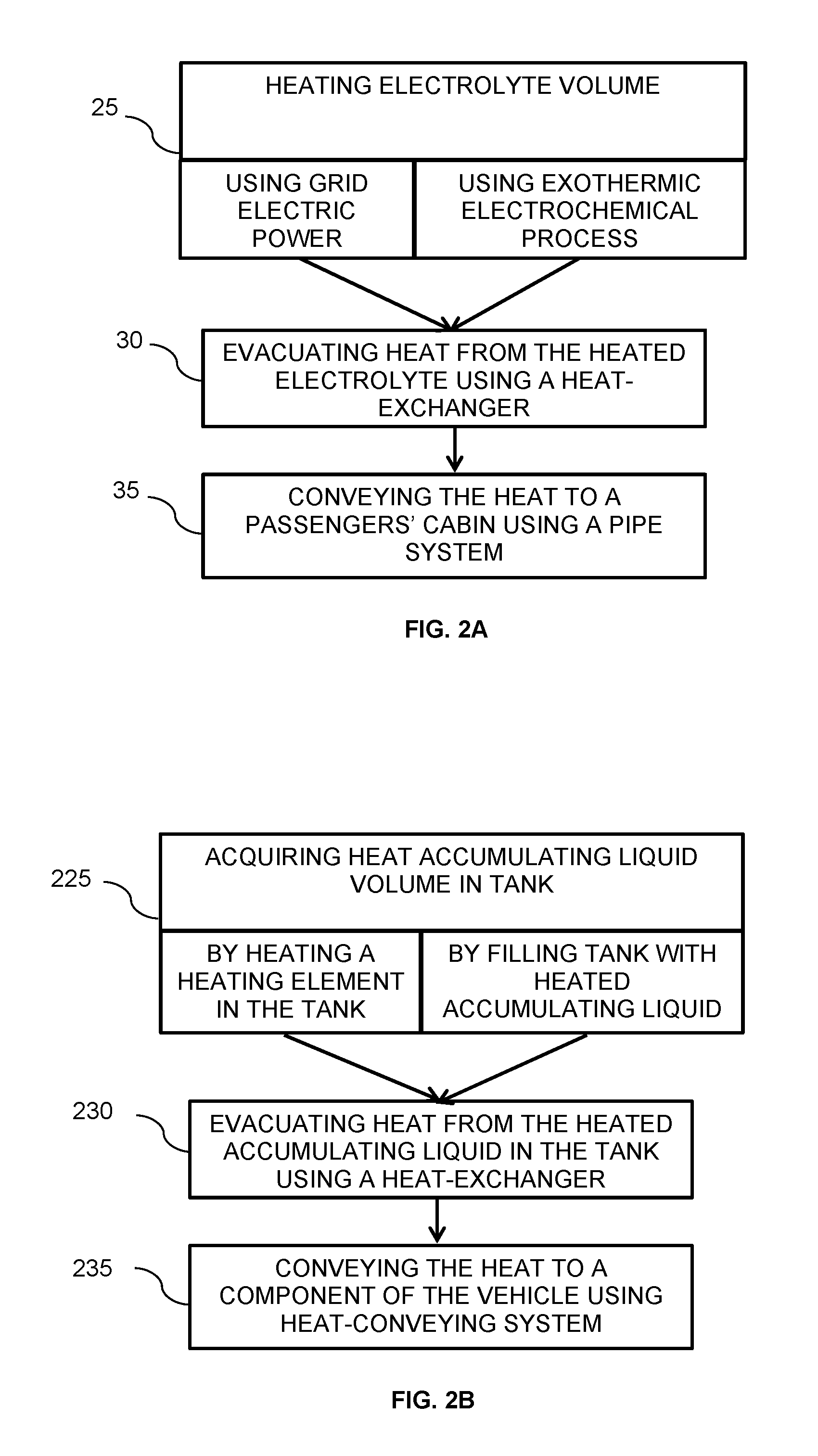
Thermal battery for heating vehicles
ActiveUS20160344082A1Fast loadingFuel and primary cellsBatteries circuit arrangementsEngineeringCold weather
A system and a method for heating a component of an electric vehicle may be particularly beneficial in cold weather places and / or during winter time. The vehicle may be primarily powered by a main battery. The system may include a supplementary battery being metal-air battery including an electrolyte, for extending the driving range of the electric vehicle and a reservoir tank for holding an electrolyte volume for the metal-air battery, the electrolyte may be heated to a desired temperature. The system may further include a heat exchanger for conveying heat from the electrolyte volume, said heat is conveyable to said passenger's cabin.
Owner:PHINERGY
Reversibly-activated nanostructured battery
A battery having a nanostructured battery electrode is disclosed wherein it is possible to reverse the contact of the electrolyte with the battery electrode and, thus, to return a battery to a reserve state after it has been used to generate current. In order to achieve this reversibility, the nanostructures on the battery electrode comprise a plurality of closed cells and the pressure within the enclosed cells is varied. In a first embodiment, the pressure is varied by varying the temperature of a fluid within the cells by, for example, applying a voltage to electrodes disposed within said cells. In a second illustrative embodiment, once the battery has been fully discharged, the battery is recharged and then the electrolyte fluid is expelled from the cells in a way such that it is no longer in contact with the battery electrode.
Owner:ALCATEL-LUCENT USA INC
Air battery system
ActiveUS20140287329A1Decrease in in inner pressureDecrease in power outputFuel and primary cellsElectrolyte moving arrangementsInternal pressureBattery system
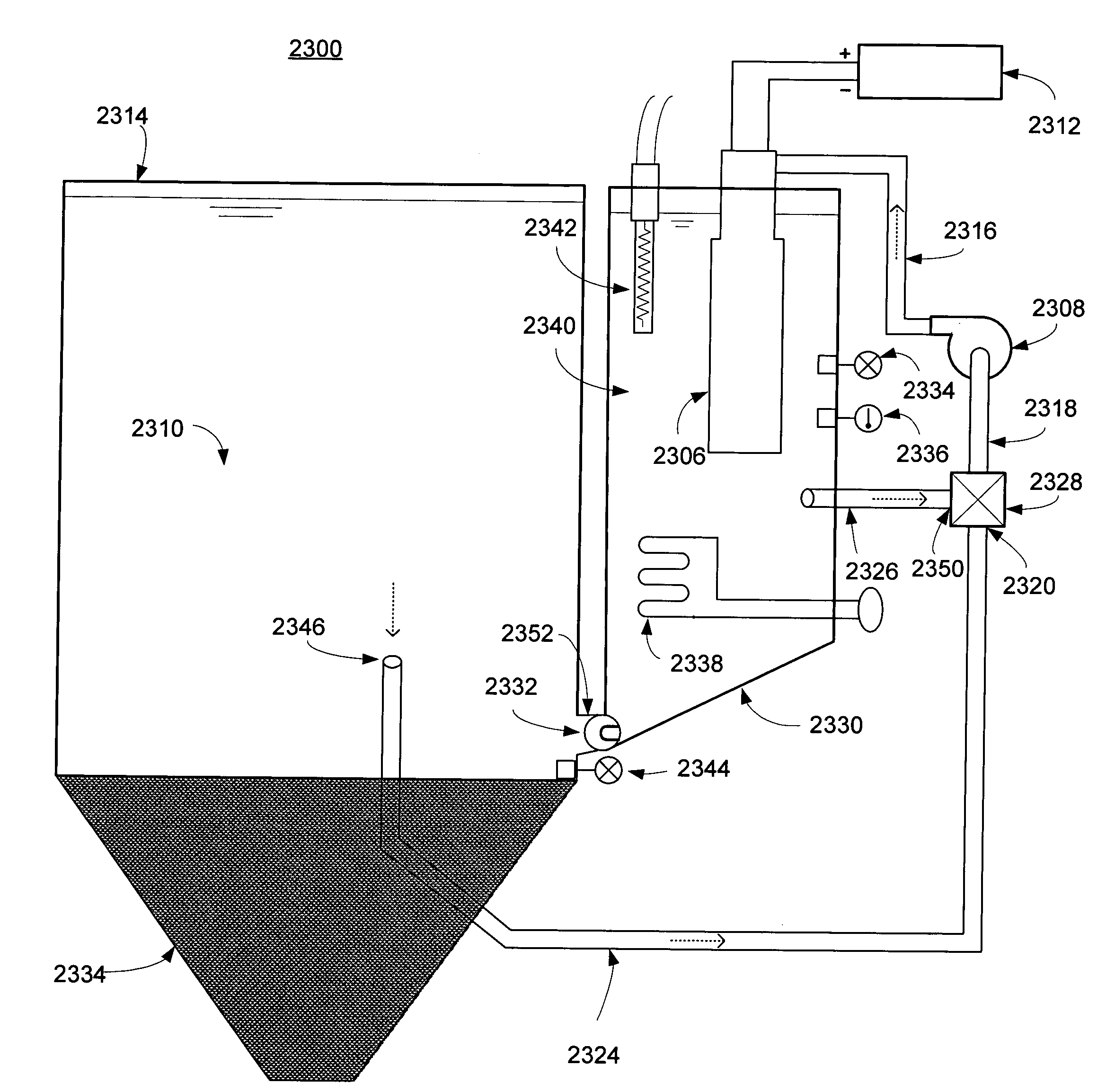
In an air battery system, a decrease in power output and an increase in inner pressure during discharge are prevented even when deposition of the reaction product increases with the discharge and the volume of the electrolytic solution increases with progress of the reaction. The air battery system includes an air battery a reservoir tank to reserve electrolytic solution to be supplied to the air battery and a reaction product sump to store reaction product produced in the air battery, the reaction product sump provided between the air battery and the reservoir tank.
Owner:NISSAN MOTOR CO LTD
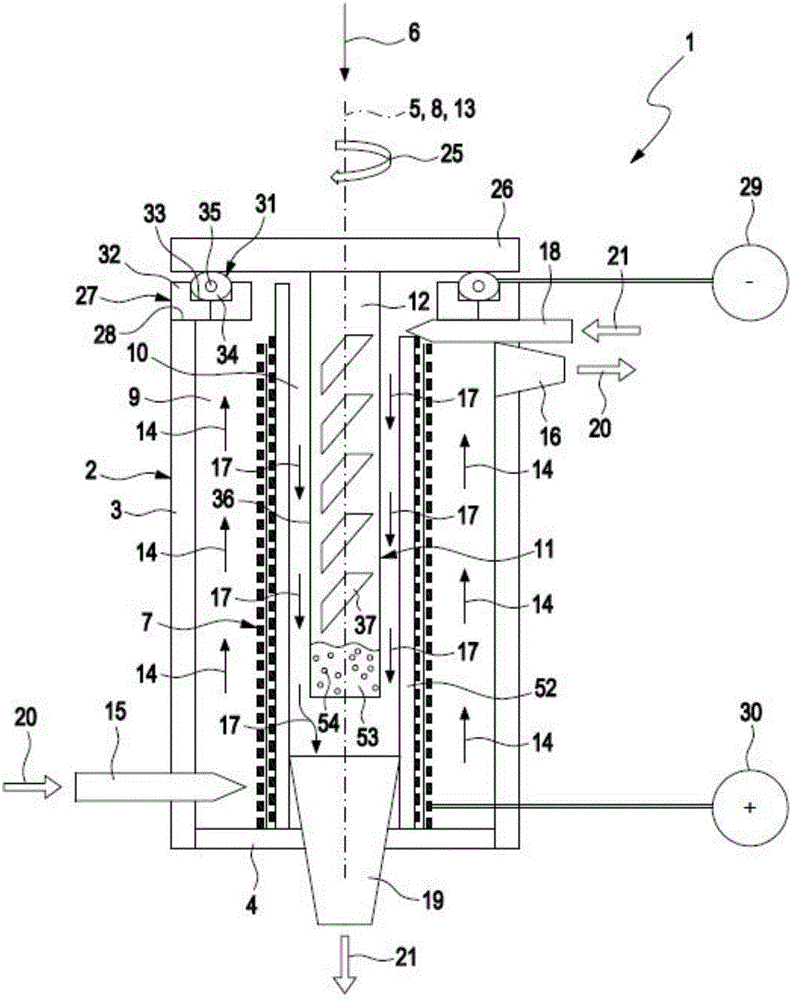
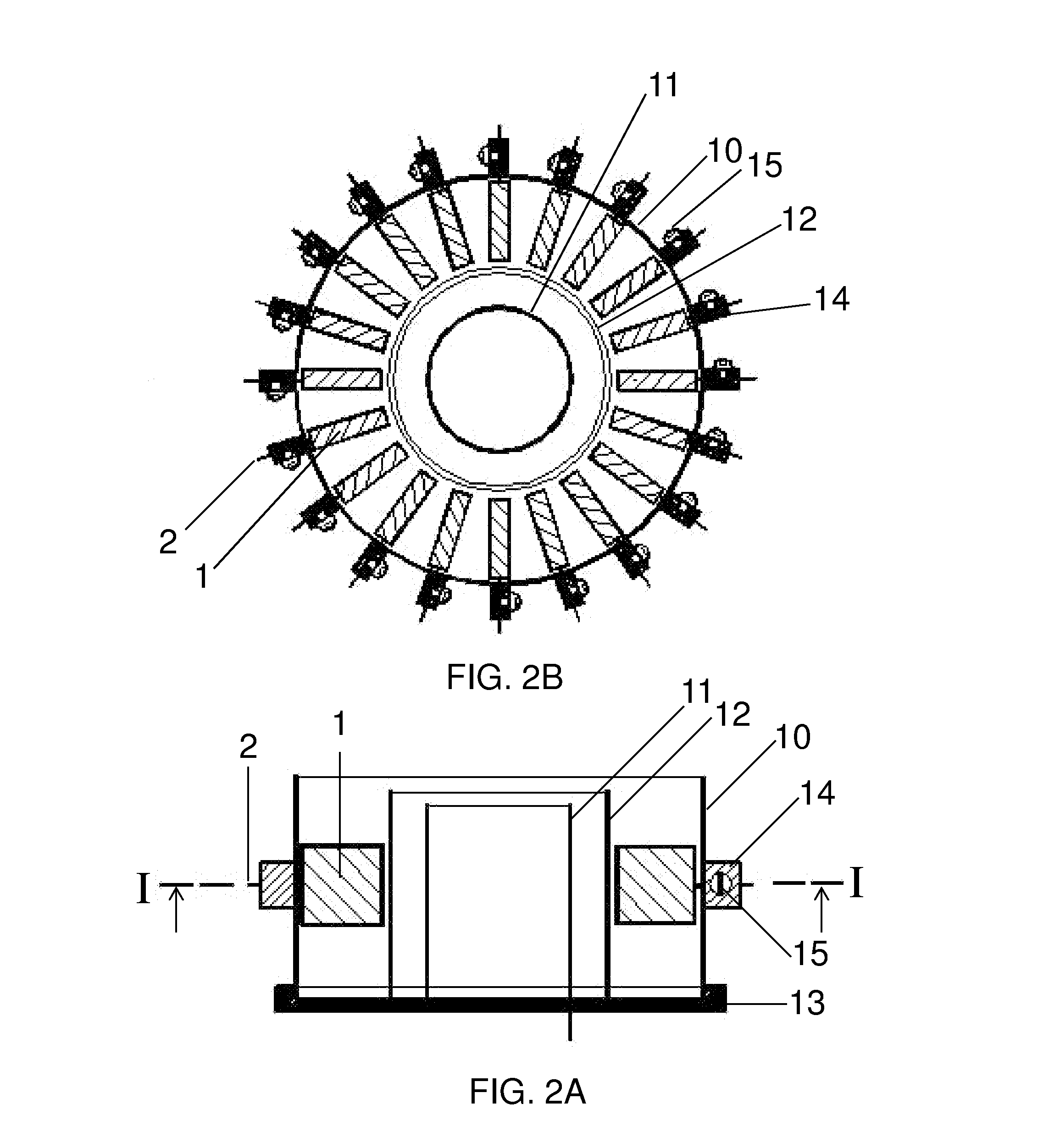
Controlled concentration electrolysis system
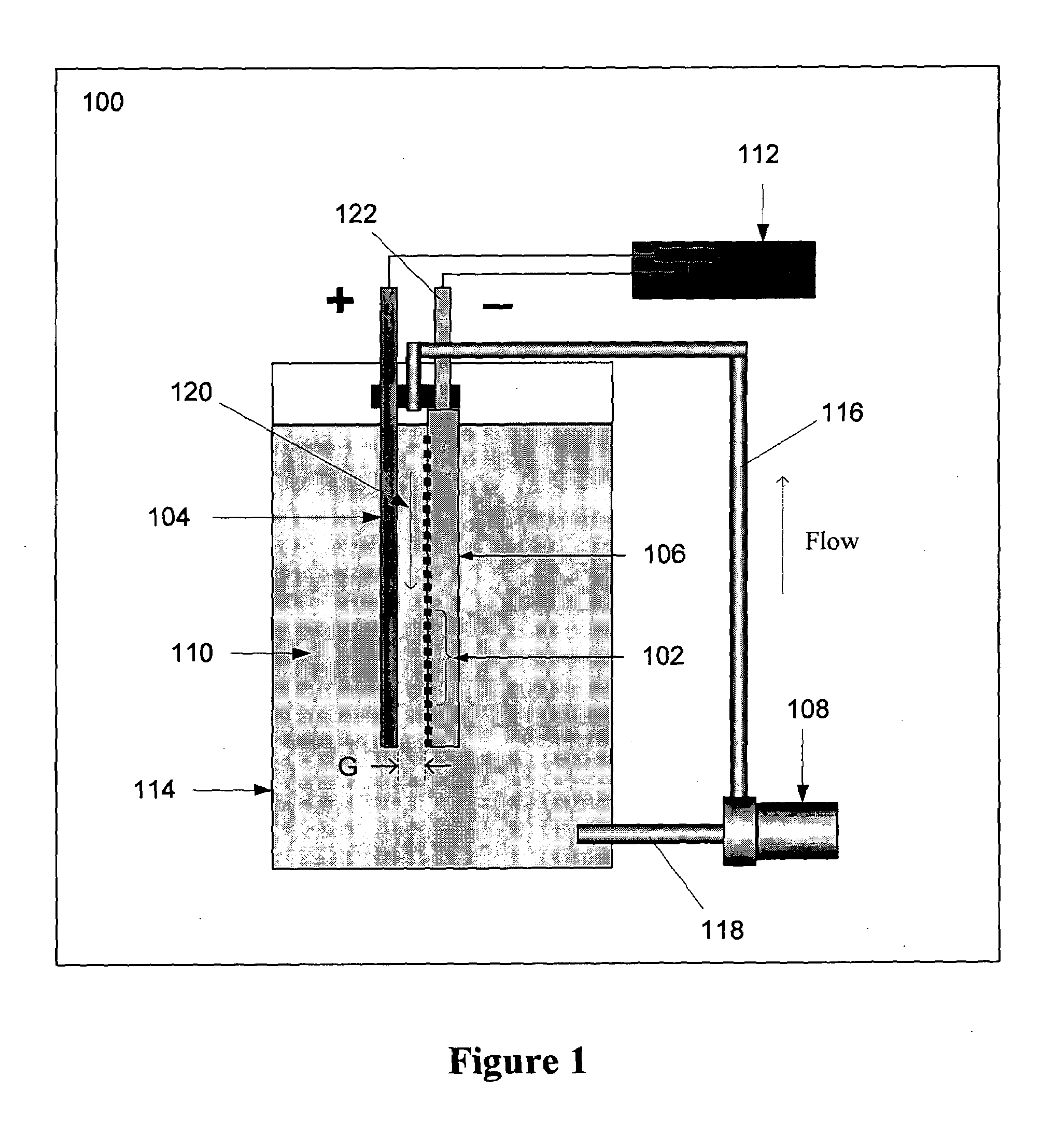
A system for maintaining a concentration range of an electroreducible metal species undergoing electrolysis within a predetermined concentration range comprises a first container containing a body of an electrolyte solution in which a metal is partially dissolved, a second container in fluid communication with the first container, the second container containing a second body of the solution, and a means for exchanging solution between the containers. The second container is configured with a means for electrolyzing, and a means for sensing the concentration of, the dissolved metal in the second body. During electrolysis, if the sensed concentration is within a predetermined range, the second body is circulated through the electrolyzing means; if the sensed concentration is outside or nearly outside the range, the solution is exchanged to maintain the concentration within the range. Optionally, the system includes a means for sensing temperature of the second body, and a means for maintaining the temperature within a predetermined range responsive to the sensed temperature by exchanging, cooling, or heating the solution comprising the second body. A method comprising these steps is also described.
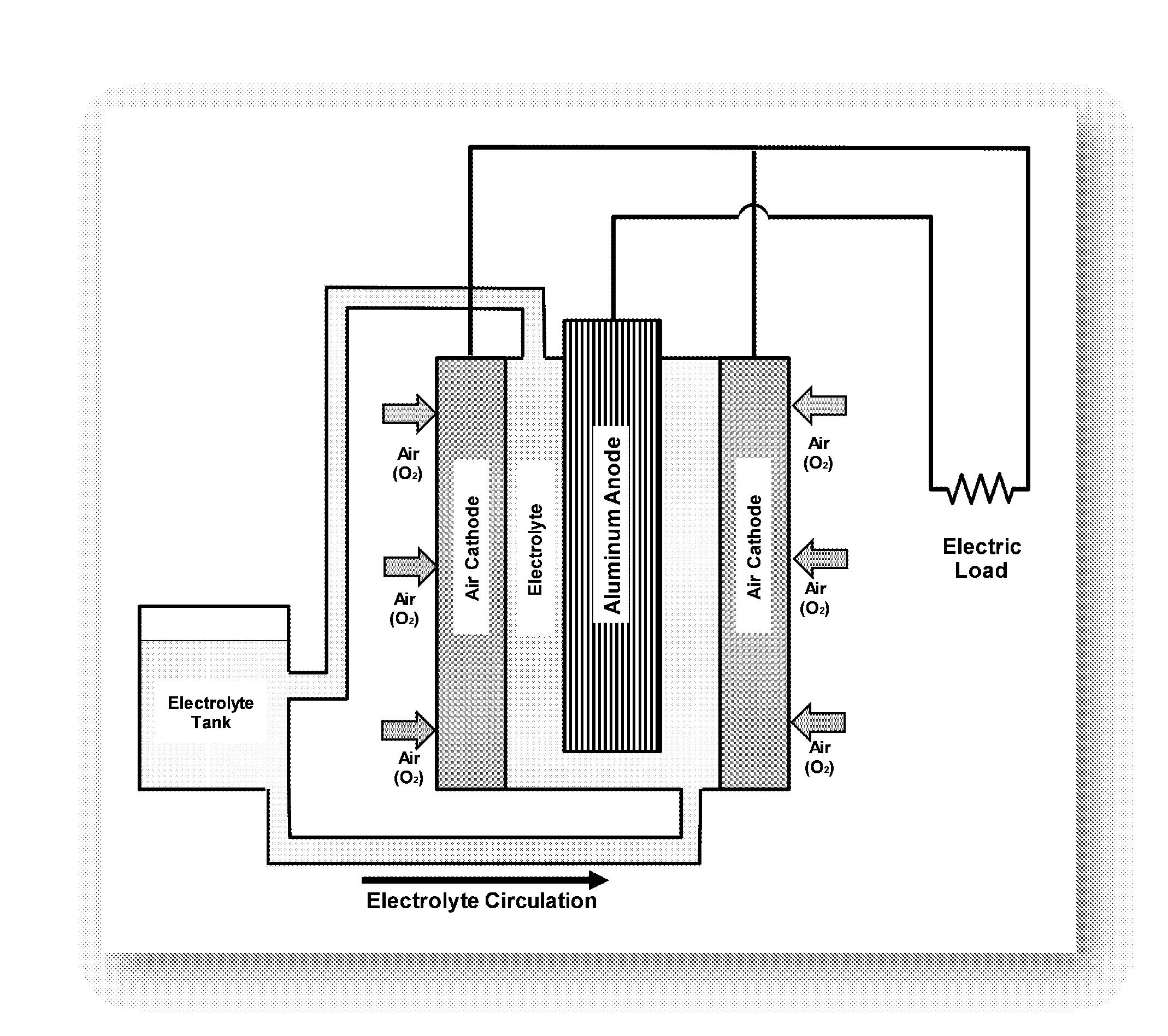
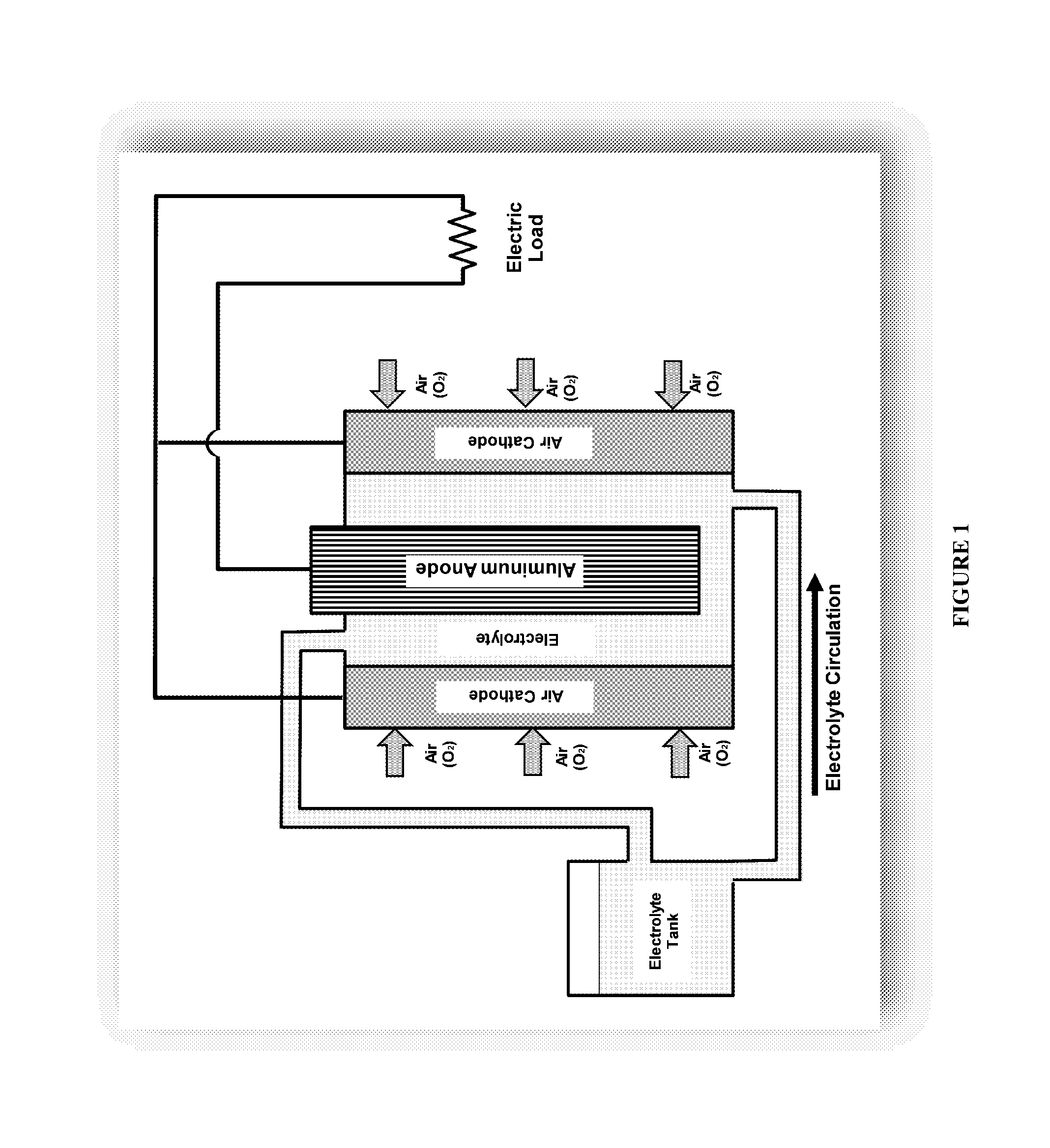
Owner:METALLIC POWER INC
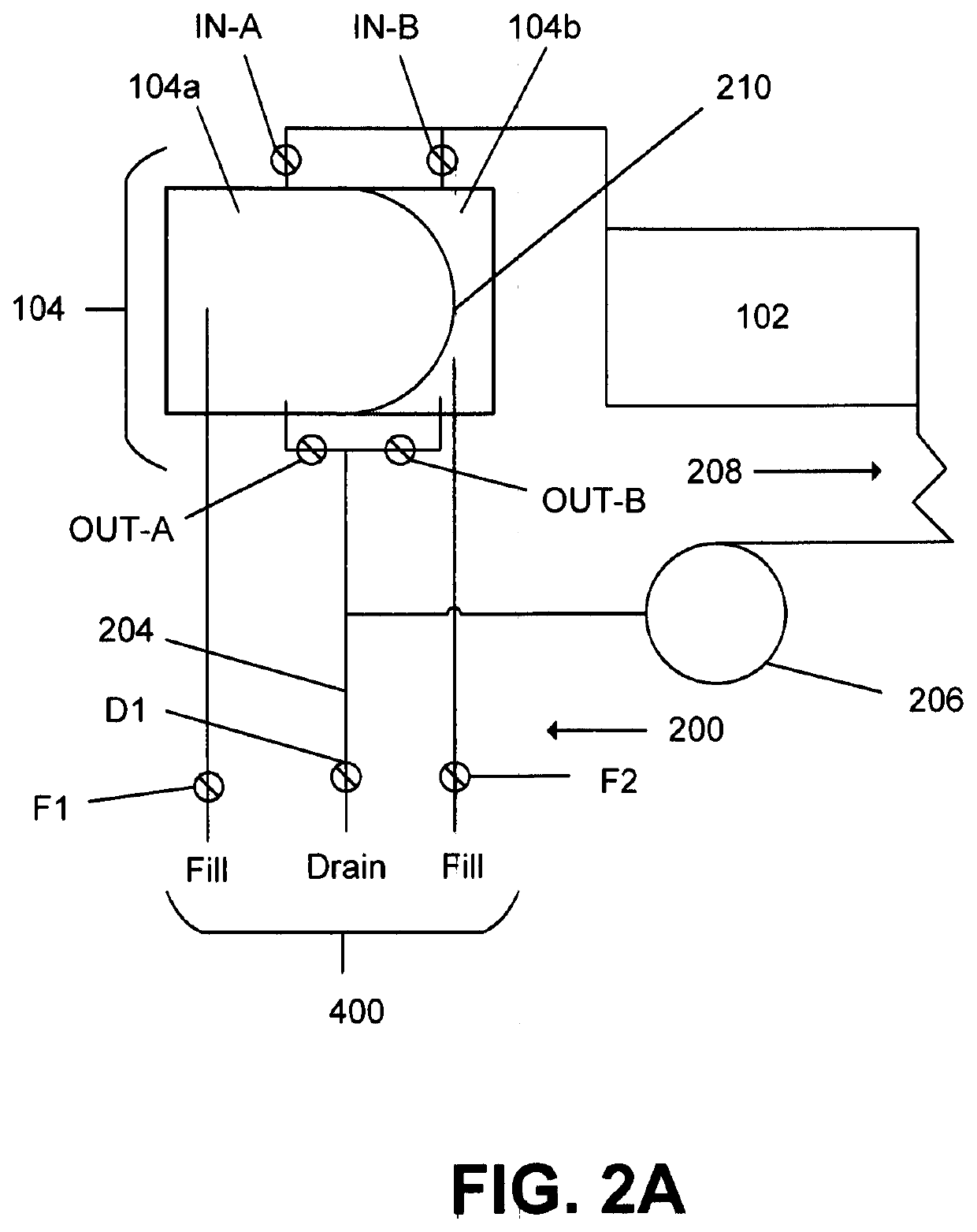
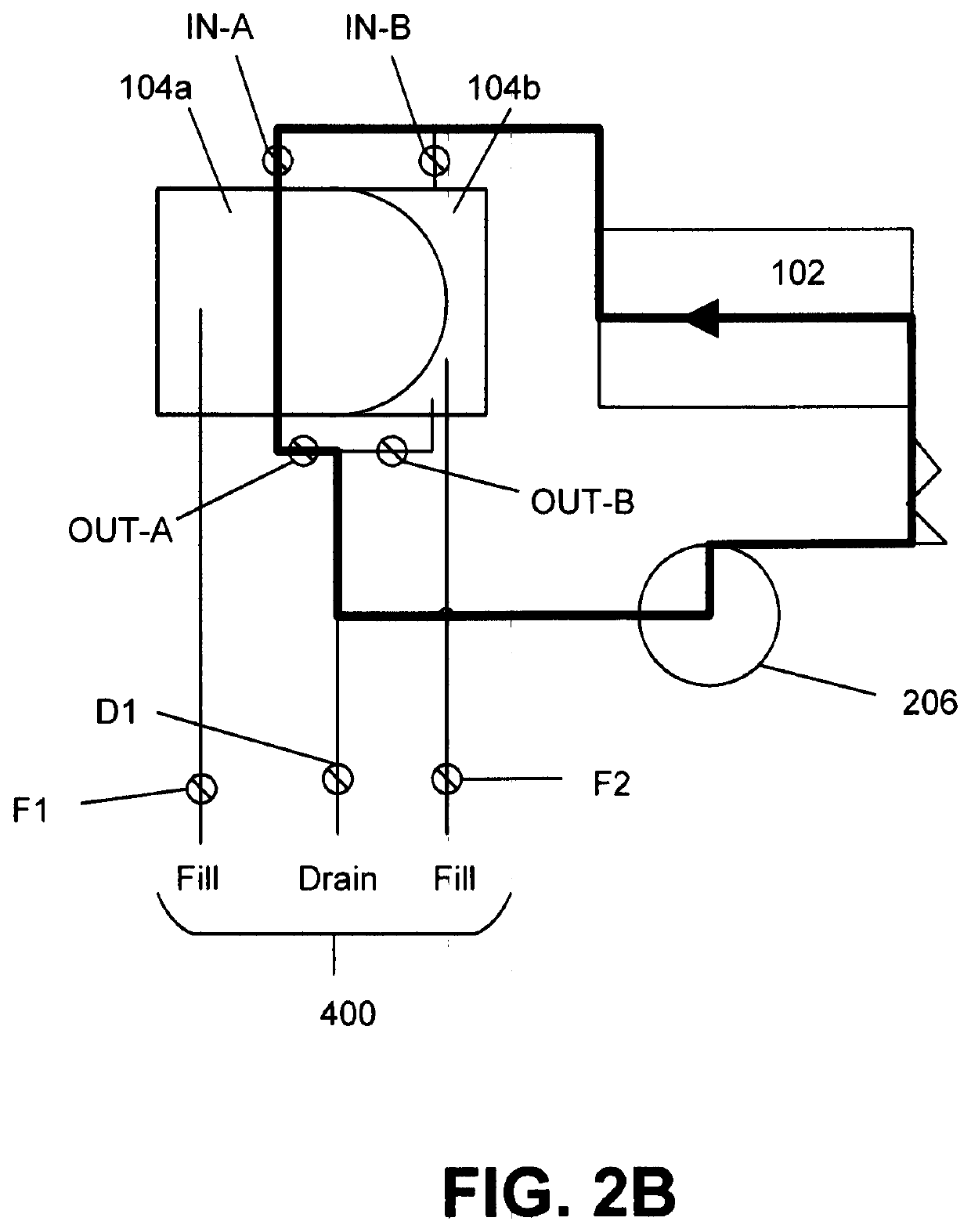
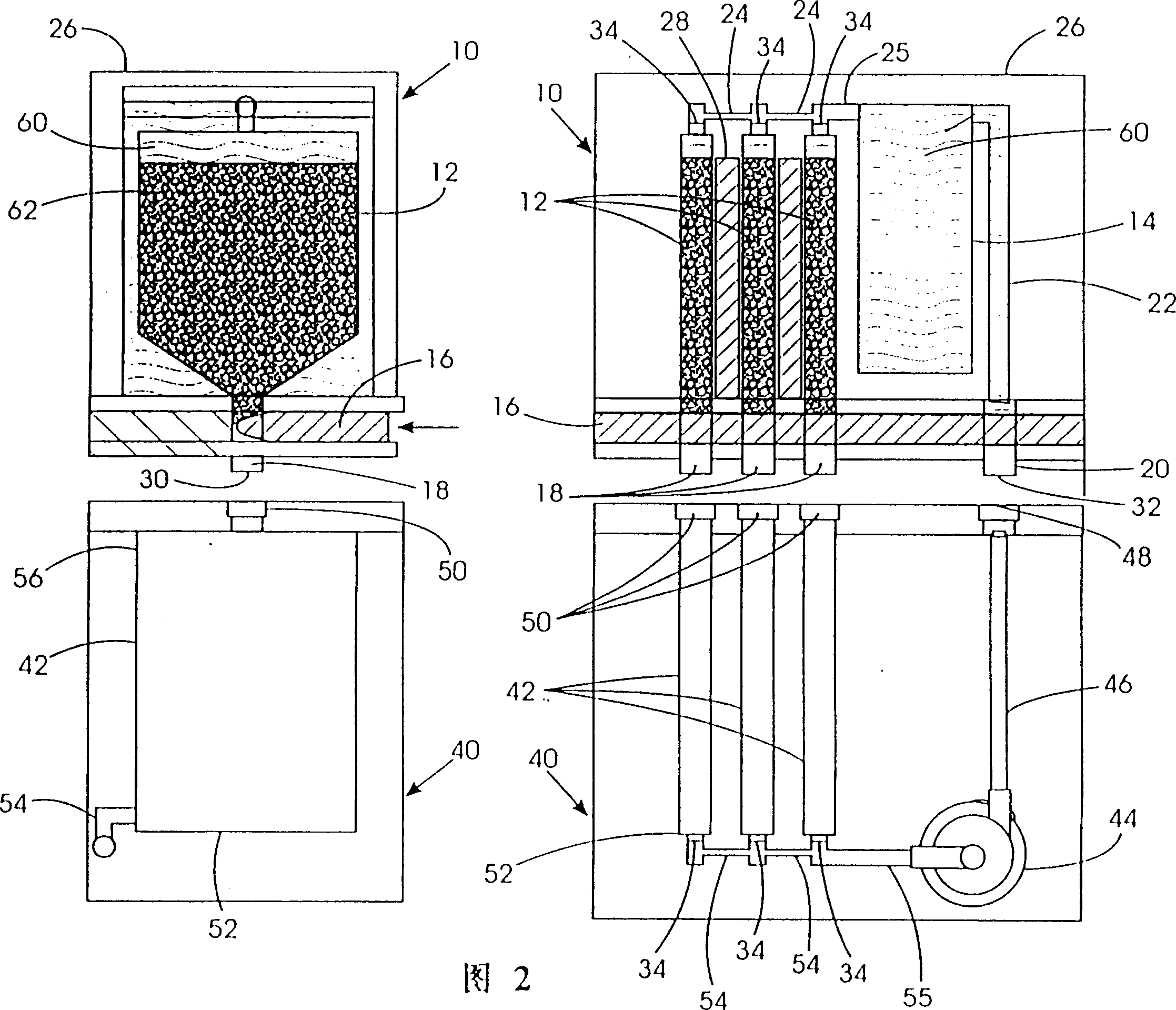
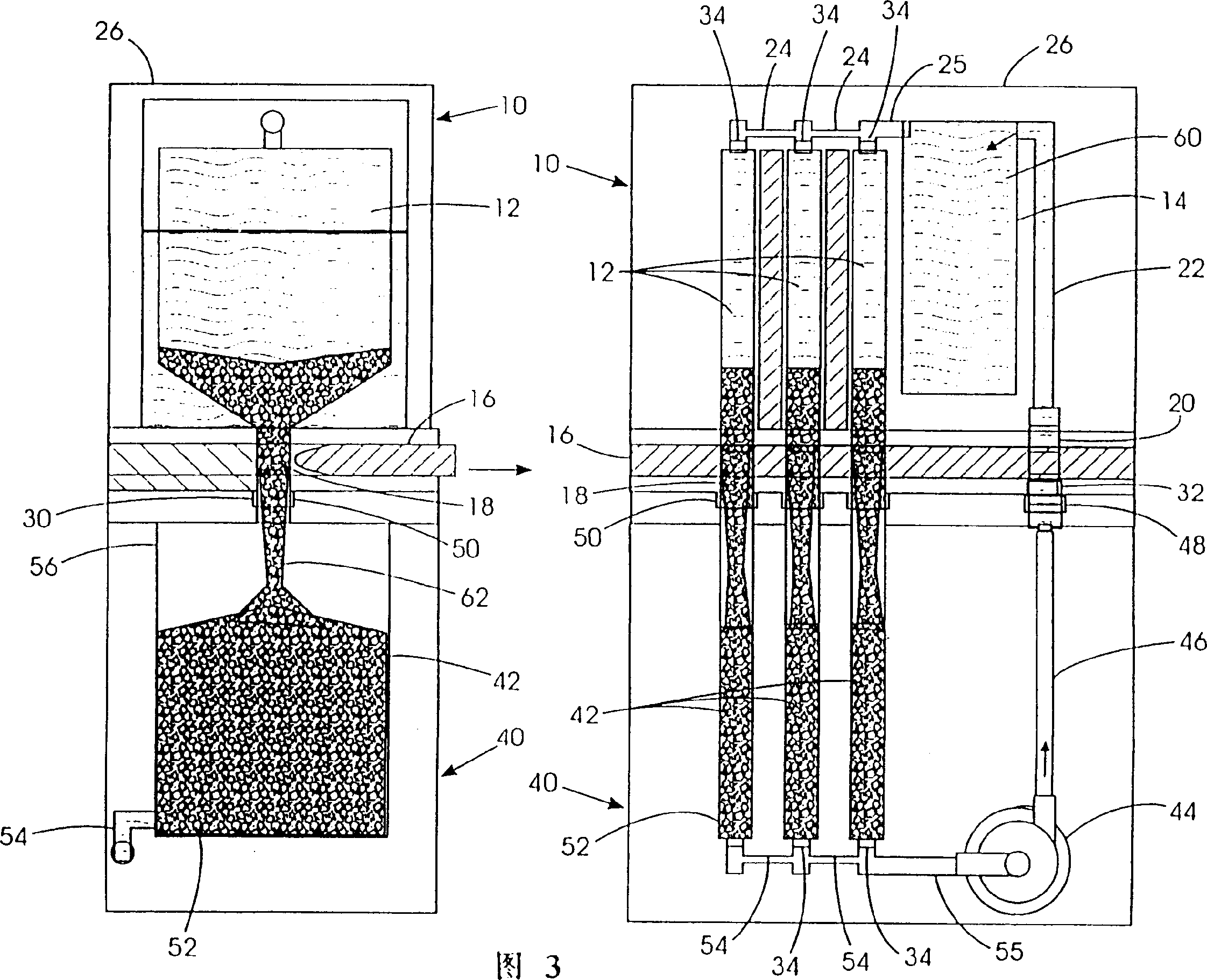
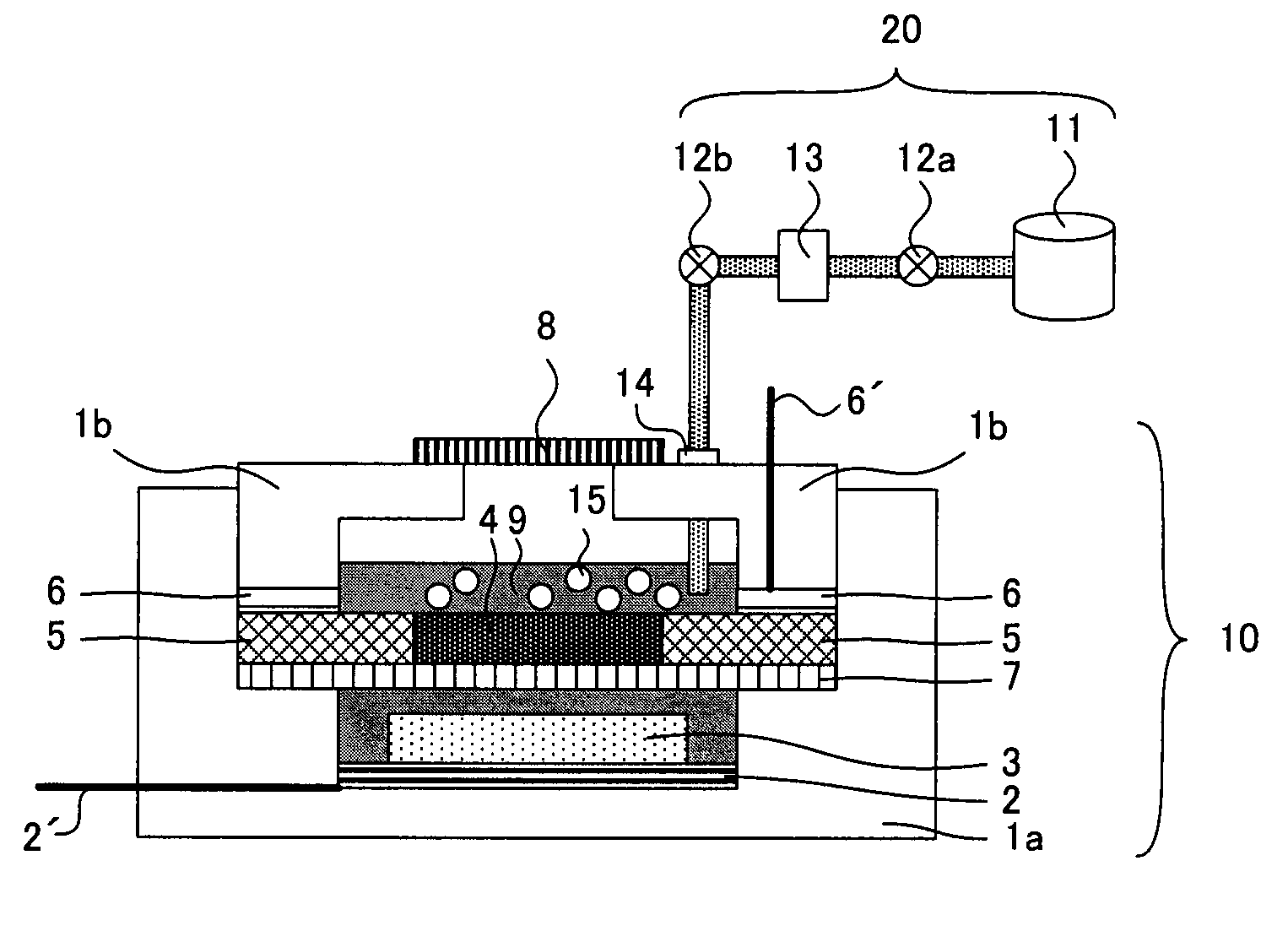
Refuelable electrochemical power source capable of maintaining in constantly full and method of using same
InactiveCN1428013AStrong electrical activityFuel and primary cellsElectrolyte moving arrangementsCell cavityEngineering
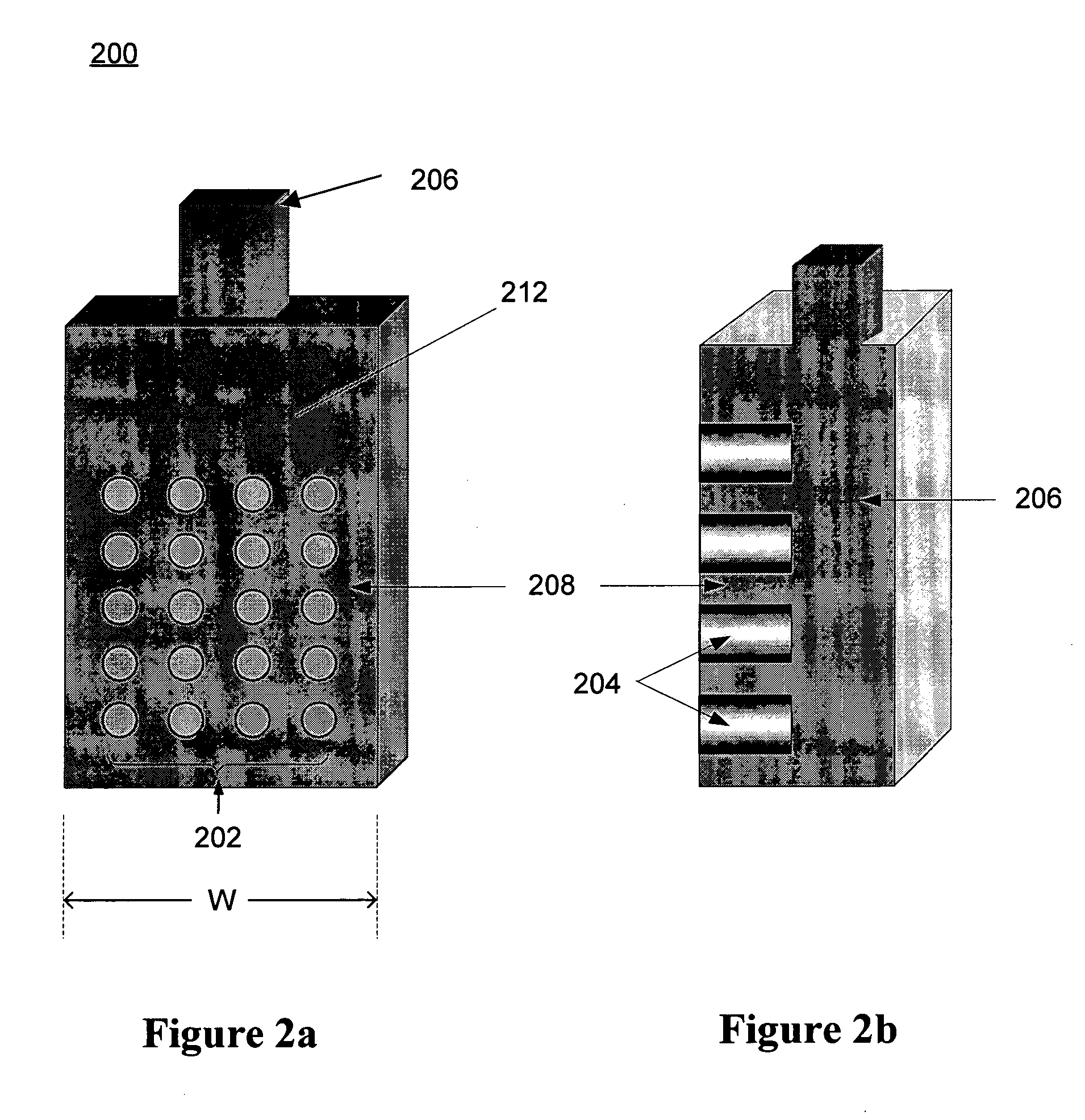
A refuelable electrochemical power source includes one or more electrochemical cells adapted to employ particulate material electrodes. The one or more cells include a cell body defining an interior cell cavity, a flow path in the cell cavity through which particulate material and fluid flow, an electroactive zone within the cell cavity, and a fluid mechanic device adjacent or within the flow path capable of filling the electroactive zone or maintaining the electroactive zone in a constantly full or maximum electroactivity condition. A method for filling or maintaining one or more cells of a refuelable electrochemical power source at a constantly full condition includes providing the above-described apparatus and constantly, periodically, or intermittently circulating particulate material and fluid through the flow path in the one or more cells, past the fluid mechanic device, so that the electroactive zone of the one or more electrochemical cells is maintained in a constantly full or maximum electroactivity condition.
Owner:METALLIC POWER INC
Metal-Air Battery with Expandable Anode
InactiveUS20160111705A1Prevent zinc oxide densificationIncrease volumetric energy densityFuel and primary cellsElectrolyte moving arrangementsElectrolysisSlurry
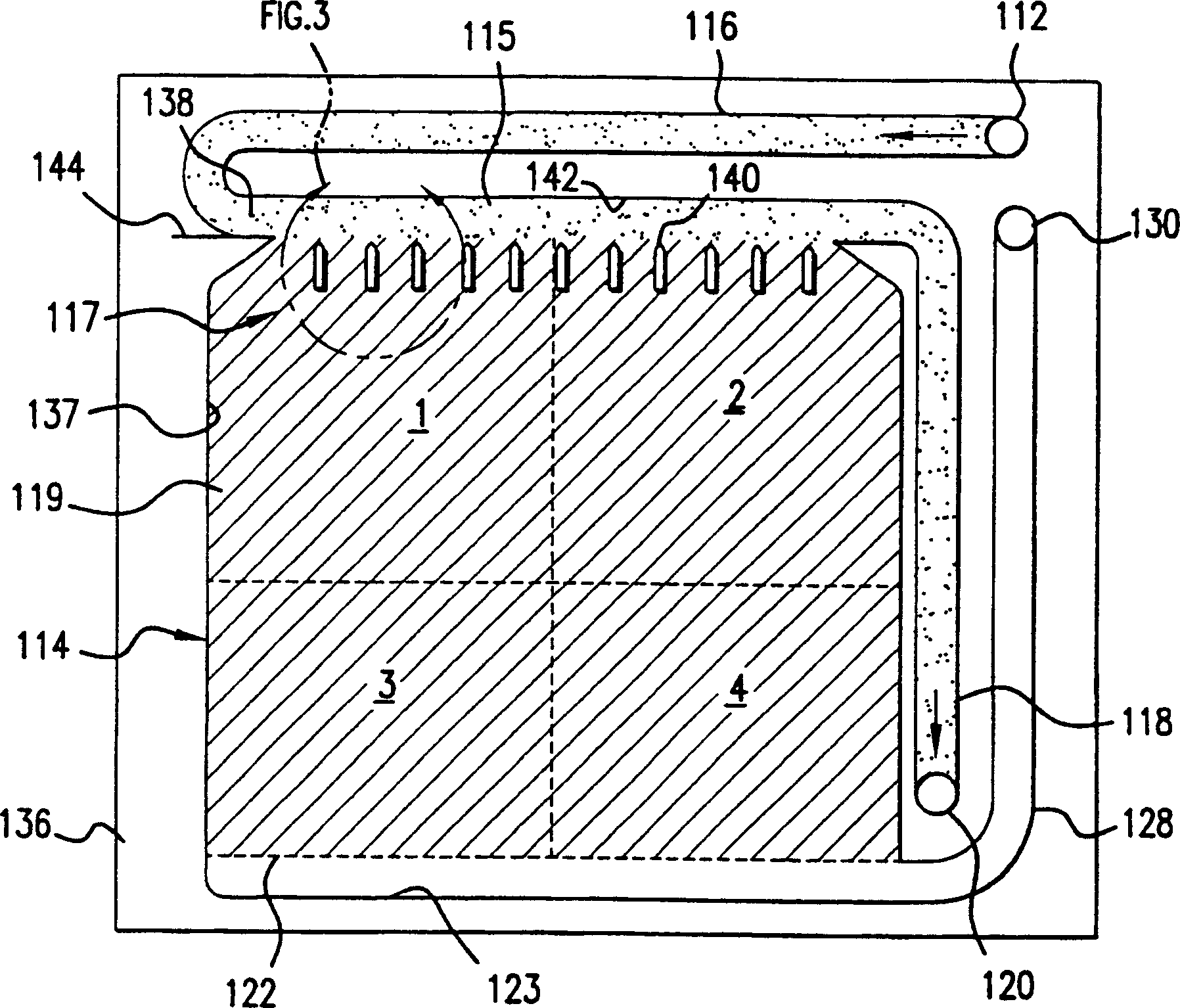
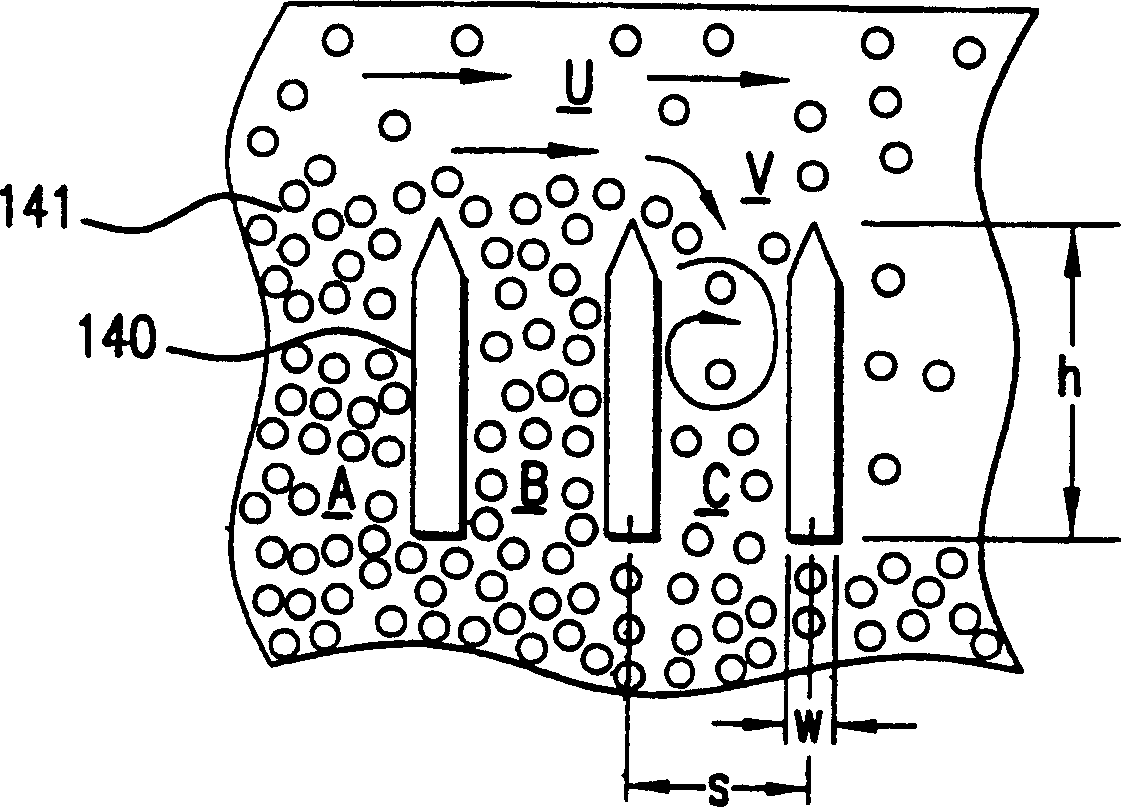
An air cathode battery is provided with a slurry anode. An anode cavity is interposed between the air cathode interior surfaces, with an anode compartment occupying the anode cavity. The anode compartment has a first wall and a second wall, one or both capable of movement. An anode current collector pouch has walls adjacent to interior surfaces of the anode compartment. A zinc slurry occupies an expandable region in the anode compartment between the anode current collector pouch and the anode compartment wall interior surfaces. The anode current collector pouch first wall and second wall contract towards each other in response to expansion in the volume of zinc slurry. In one aspect, the anode compartment first and second walls expand away from each other in response to expansion in the volume of zinc oxide. A replenishable electrolyte source may be used to provide electrolyte to the anode cavity.
Owner:SHARP LAB OF AMERICA INC
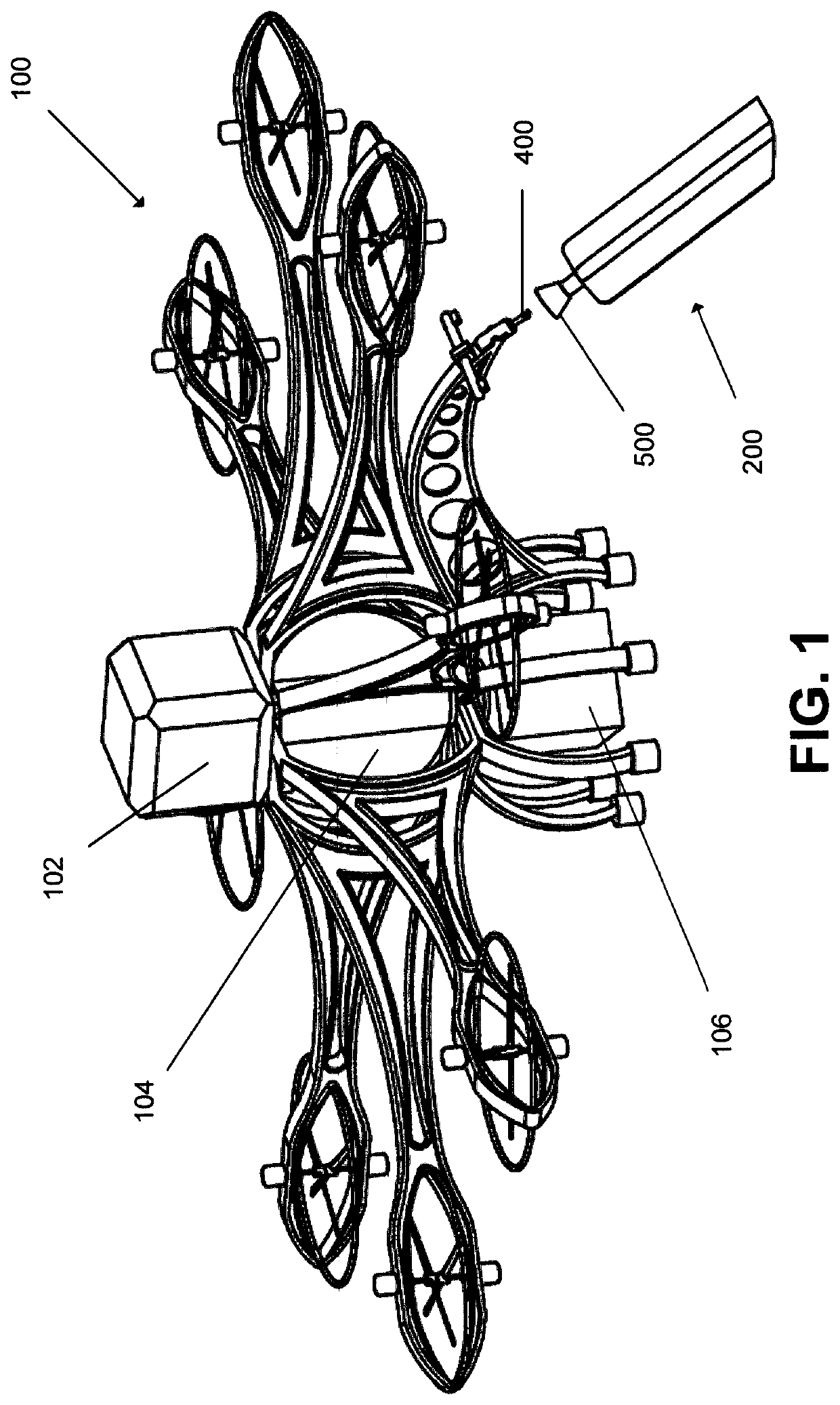
Rapid electrolyte replenishment system for aerial drones
PendingUS20210202959A1Extended flight timeExtended range timeLiquid handling installationsPower plant fuel tanksAtmospheric sciencesFlight time
A metal air battery electrolyte replenishment system comprised of a base station with docking receptor apparatus and matching docking probe on a flying drone. The probe onboard the drone has a sensor that guides the drone to connect with the electrolyte docking receptor on the base station. The drone uses the probe to obtain fresh electrolyte and simultaneously expel spent electrolyte into the base station while still in flight or during a brief landing. Rapid exchange of the electrolyte allows for extended range and flight time without penalty of onboard electrolyte reconditioning system and its associated weight.
Owner:ALUMAPOWER CORP
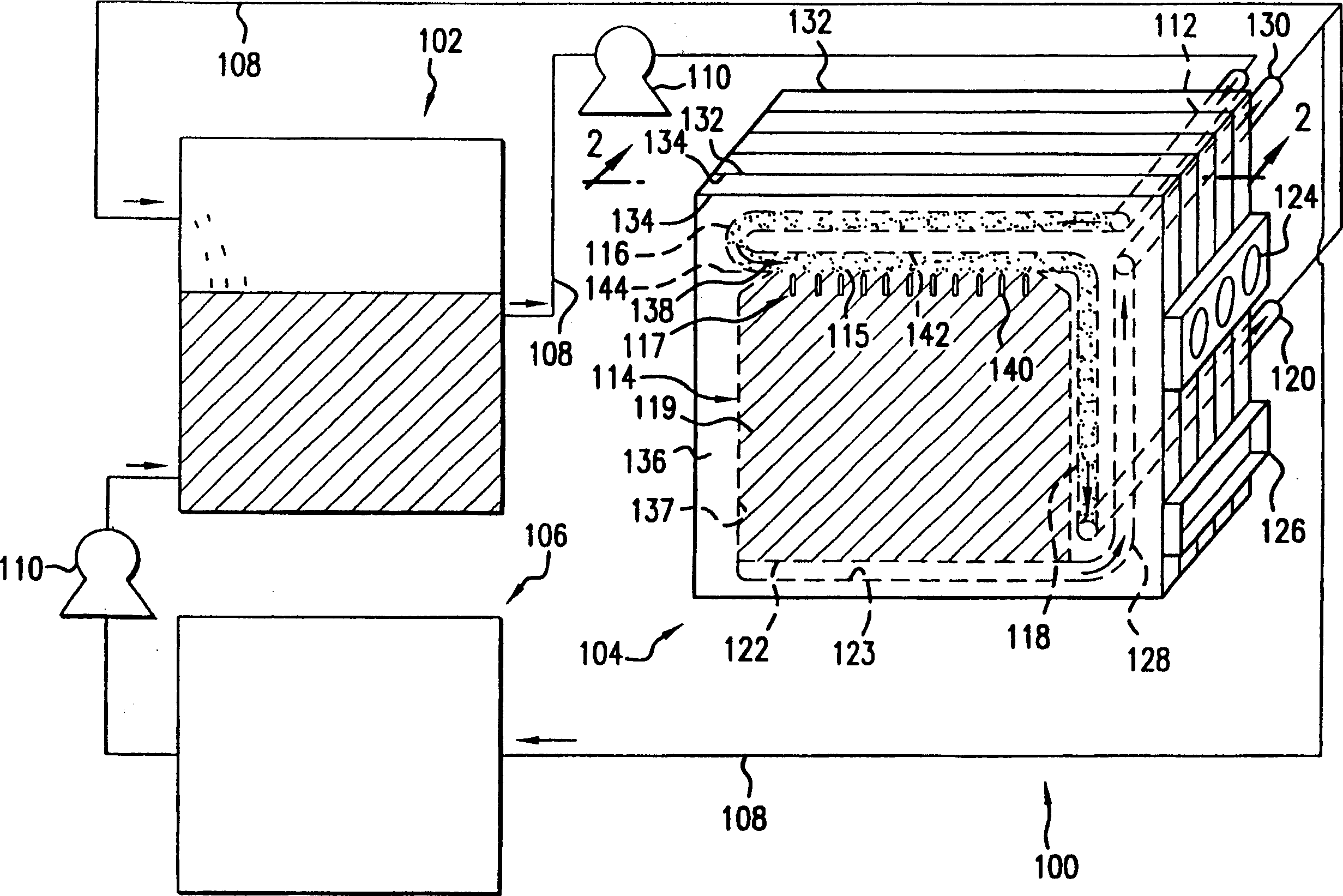
Method and apparatus for refilling fuel to electrochemical power source
A transportable container (10) for refueling a refuelable battery (40) includes a case (26), an electrolyte reservoir (14) within the case (26), an electrolyte reservoir (14) within the case (26), afirst valve connected to the electrolyte reservoir (26), a fuel compartment (12) within the case (26), a second valve connected to the fuel compartment (12), and a conduit (24) connected to the electrolyte reservoir and the fuel compartment (12), when the transportable container (10) is attached to a refuelable battery (40), a closed flow circuit for the circulation of electrolyte (60) is defined. Fuel particles (62) and electrolyte (60) are fed from the transportable container (10) into the refuelable battery (40). When the refuelable battery (40) is discharged, the transportable container (10), containing spent electrolyte and reaction products, is detached from the refuelable battery (40).
Owner:METALLIC POWER INC
Conformable battery
InactiveUS20090042096A1Increase surface areaImprove heat transfer performanceElectrolyte moving arrangementsMoving electrode arrangementsInternal pressurePower mode
A conformable battery wherein the outer casing has an upper face plate, a lower face plate, and at least one perimetric wall, and an interior of the battery comprising a grid of walls extending from the upper face plate to the lower face plate and connecting to the at least one perimetric wall, thereby dividing the interior of the battery into at least two compartments and increasing the battery's structural stiffness and ability to sustain increased internal pressure. Each compartment contains an electrochemically active plate stack, and a network of electrical conductors provides electrical connection between the plate stacks in each of the compartment. The battery may further comprise a reservoir containing an acid additive which, when released into each of the compartments, shifts the battery from a low power mode into a high power mode.
Owner:MCDERMOTT PATRICK P
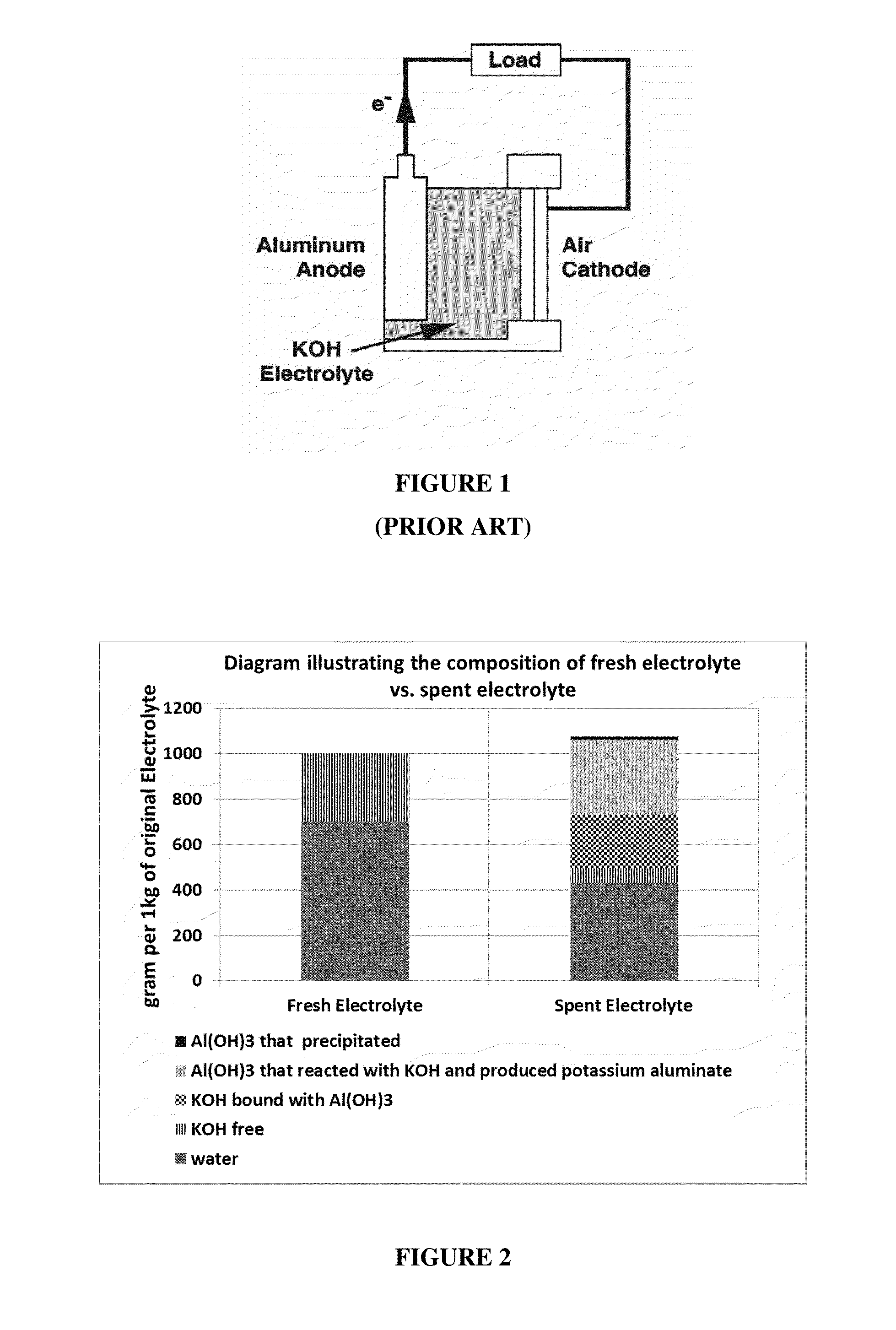
Method for regenerating alkaline solutions
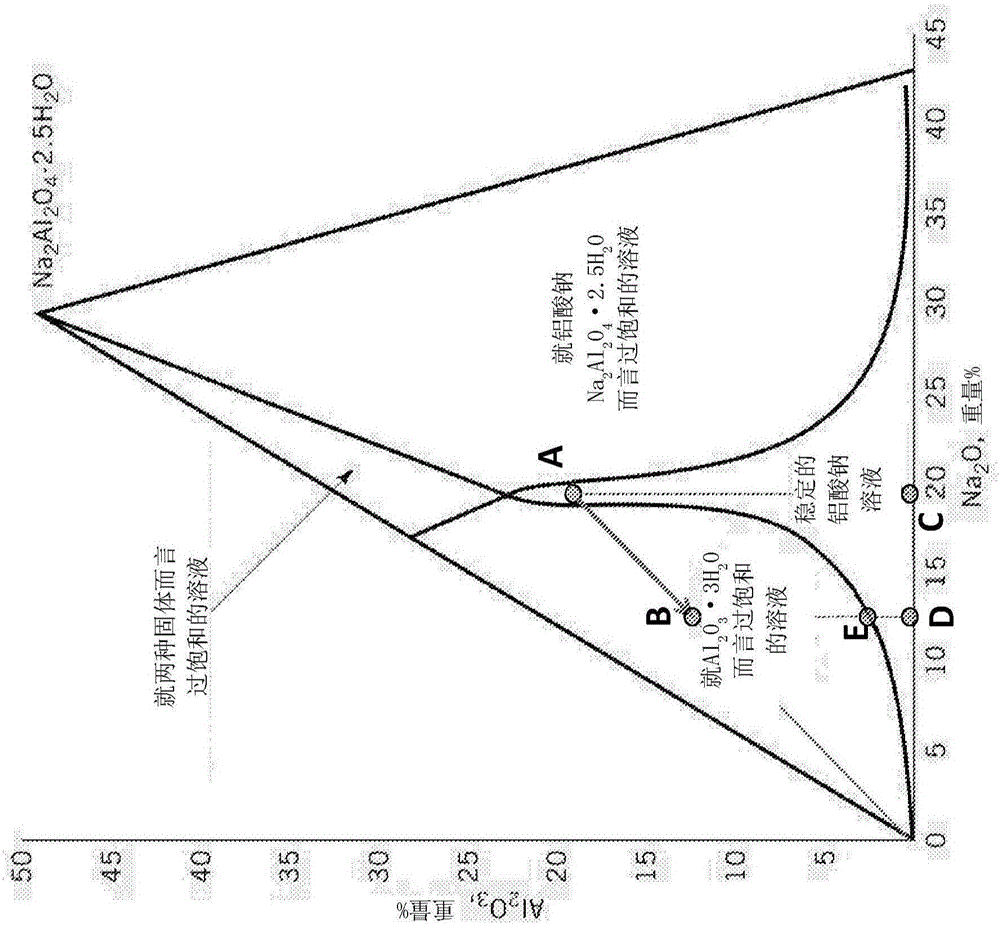
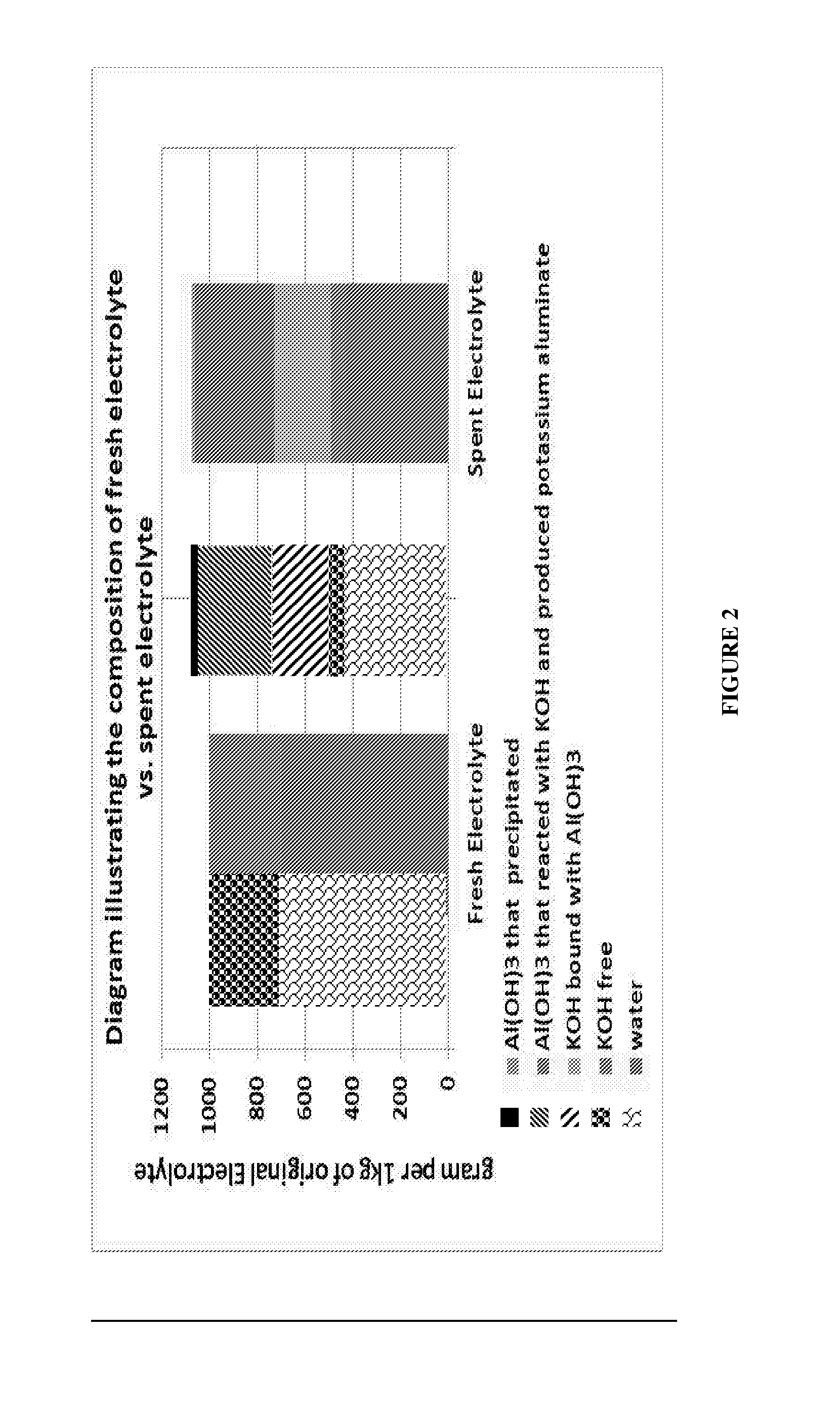
This invention relates to the regeneration of spent alkaline solutions, for example, alkaline electrolyte solutions used in metal / air batteries, specifically in aluminum / air batteries. The invention provides methods and systems to regenerate alkaline electrolyte solutions by adding water and optionally other solvents to spent electrolyte solutions, thus precipitating metal hydroxides from the spent electrolyte solution.
Owner:PHINERGY
Metal-air battery
ActiveCN106463663AExtended service lifeEasy to change and relatively fastFuel and primary cellsFuel and secondary cellsEngineeringMetal
The invention relates to a metal-air battery (1), comprising a housing (2), a cathode (7), which is arranged between an air space (9) and an electrolyte space (10) in the housing (2), a metal anode (11), which is arranged in the electrolyte space (10), an air path (14) leading through the housing (2), which air path leads from an air inlet (15) of the housing (2) to an air outlet (16) of the housing (2), an air supply device (20) for producing an air flow, which follows the air path (14) and impinges on the cathode (7), an electrolyte path (17) leading through the housing (2), which electrolyte path leads from an electrolyte inlet (18) of the housing (2), which electrolyte inlet is fluidically connected to the electrolyte space (10), to an electrolyte outlet (19) of the housing (2), which electrolyte outlet is fluidically connected to the electrolyte space (10), and an electrolyte supply device (21) for producing an electrolyte flow, which follows the electrolyte path (17) and impinges on the anode (11) and the cathode (7).
Owner:MAHLE INT GMBH
Air battery system
InactiveUS8871395B2Avoid internal resistanceFuel and primary cellsFuel and secondary cellsConductive materialsEngineering
An air battery system may include an air battery cell containing an air cathode, an anode, a separator, and an oxygen gas supply for supplying an oxygen gas by bubbling to a liquid electrolyte. The air cathode may include an air cathode layer containing a conductive material, and an air cathode current collector for collecting current of the air cathode layer. The anode may include an anode layer containing an anode active material which stores and releases a metal ion, and an anode current collector for collecting current of the anode layer. The separator may be provided between the air cathode layer and the anode layer, wherein the air cathode layer and the anode layer may be constantly filled with the liquid electrolyte at a time of a change in a volume of the electrode caused by a discharge or a discharge and charge.
Owner:TOYOTA JIDOSHA KK
Large-Scale Metal-Air Battery with Slurry Anode
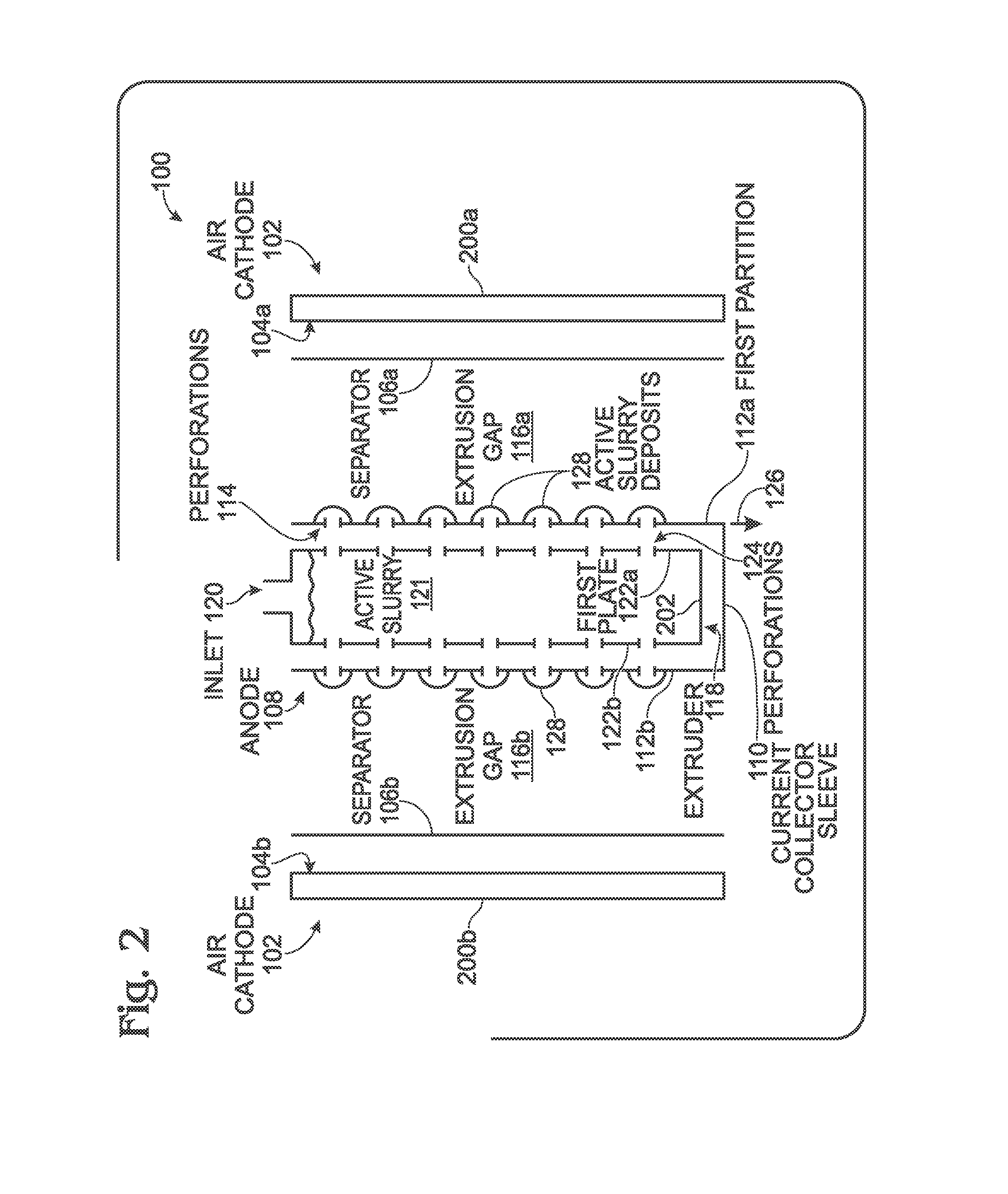
ActiveUS20150207191A1High power outputReduce resistanceFuel and primary cellsFuel and secondary cellsElectricitySlurry
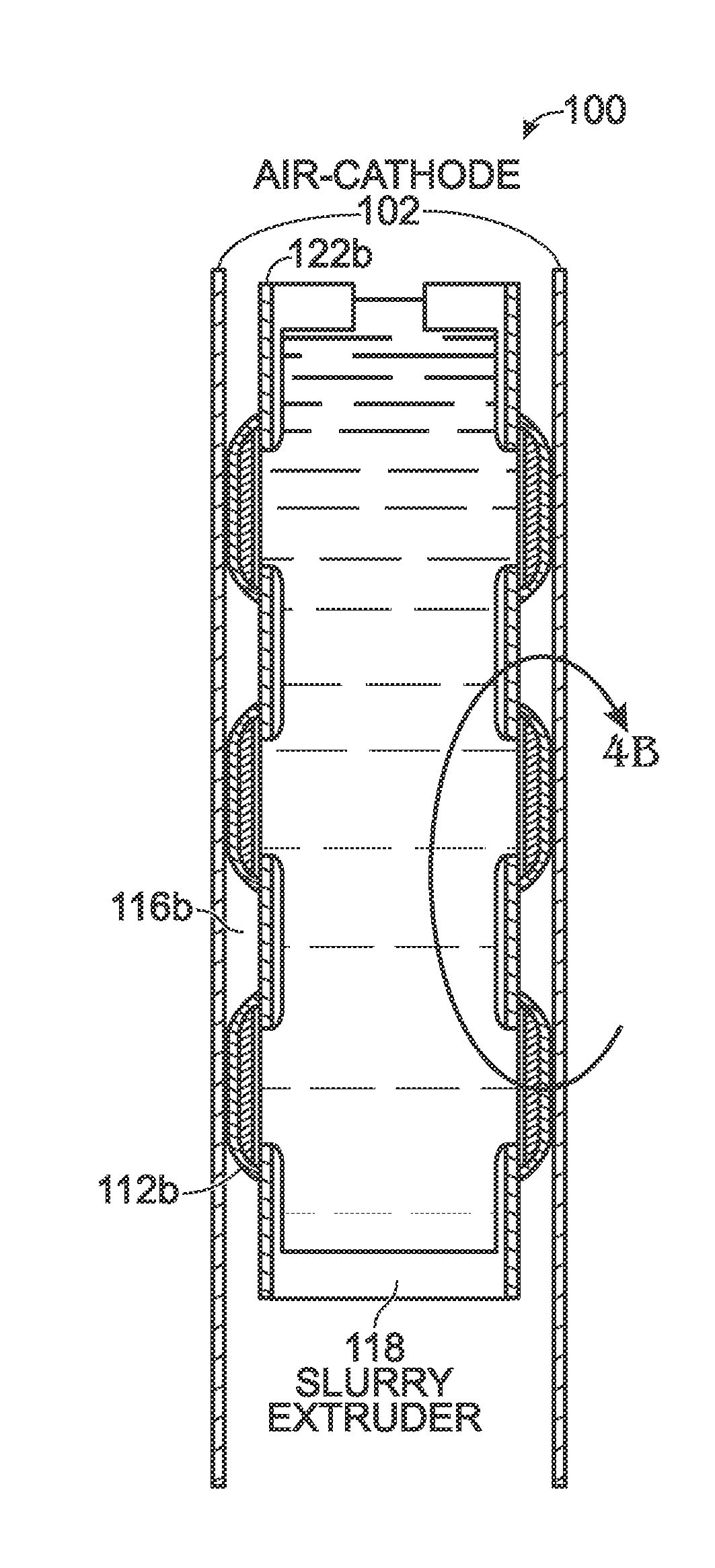
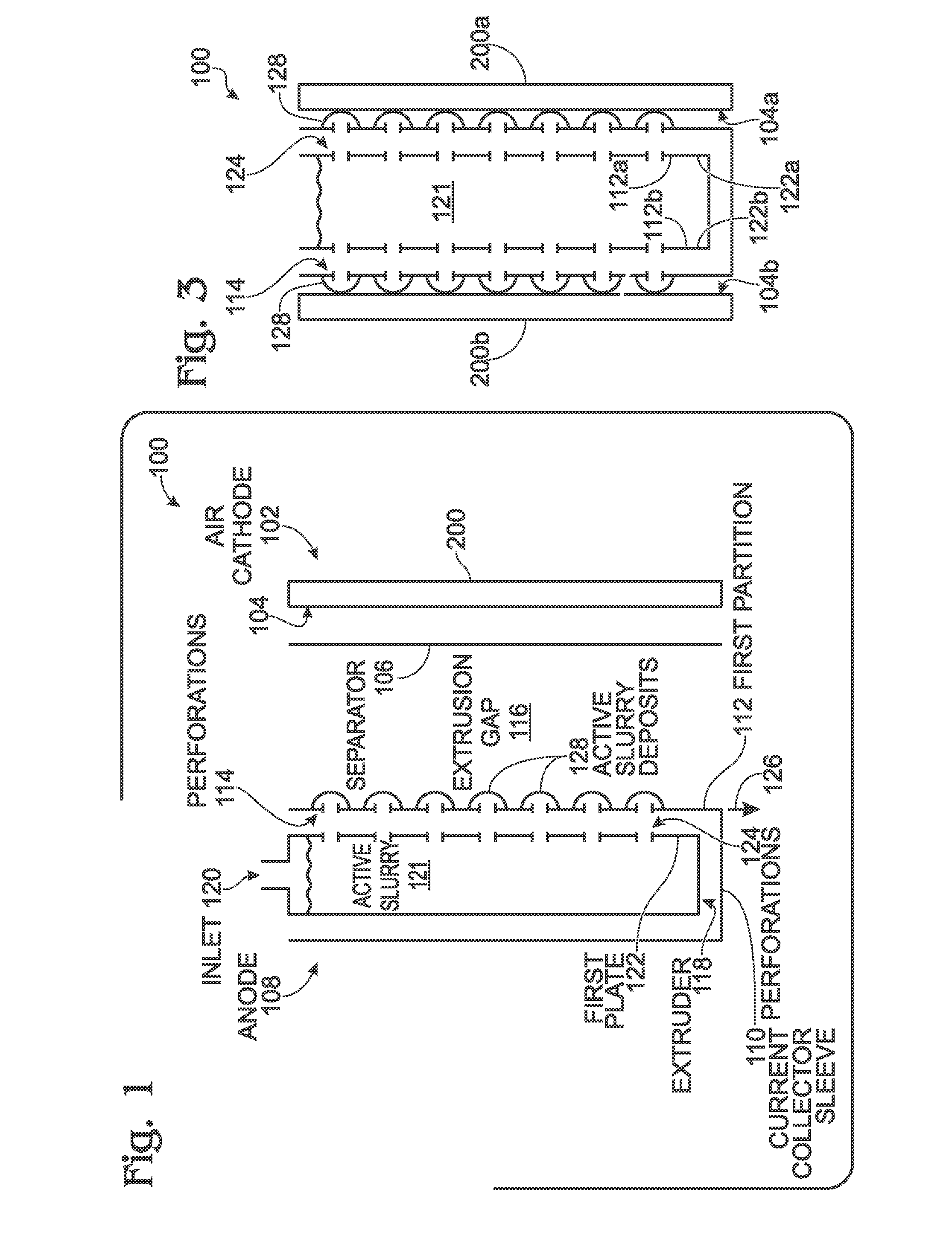
A battery and an associated method are provided for generating power using an air cathode battery with a slurry anode. The method provides a battery with an air cathode separated from an anode current collector by an electrically insulating separator and an extrusion gap. The anode current collector extruder has a first plate with a plurality of slurry outlet perforations, and a sleeve having a first partition immediately adjacent to the extruder first plate, with a plurality of slurry inlet perforations. Active slurry is provided under pressure to an extruder inlet, and the extruder first plate slurry outlet perforations are selectively aligned with sleeve first partition slurry inlet perforations. Active slurry deposits are formed in the extrusion gap to mechanically charge the battery. In the discharge position, the sleeve moves so that the perforations no longer align, and slurry in the extruder is isolated from slurry in the extrusion gap.
Owner:SHARP KK
System for generating electrical power from flue gas and captured carbon dioxide
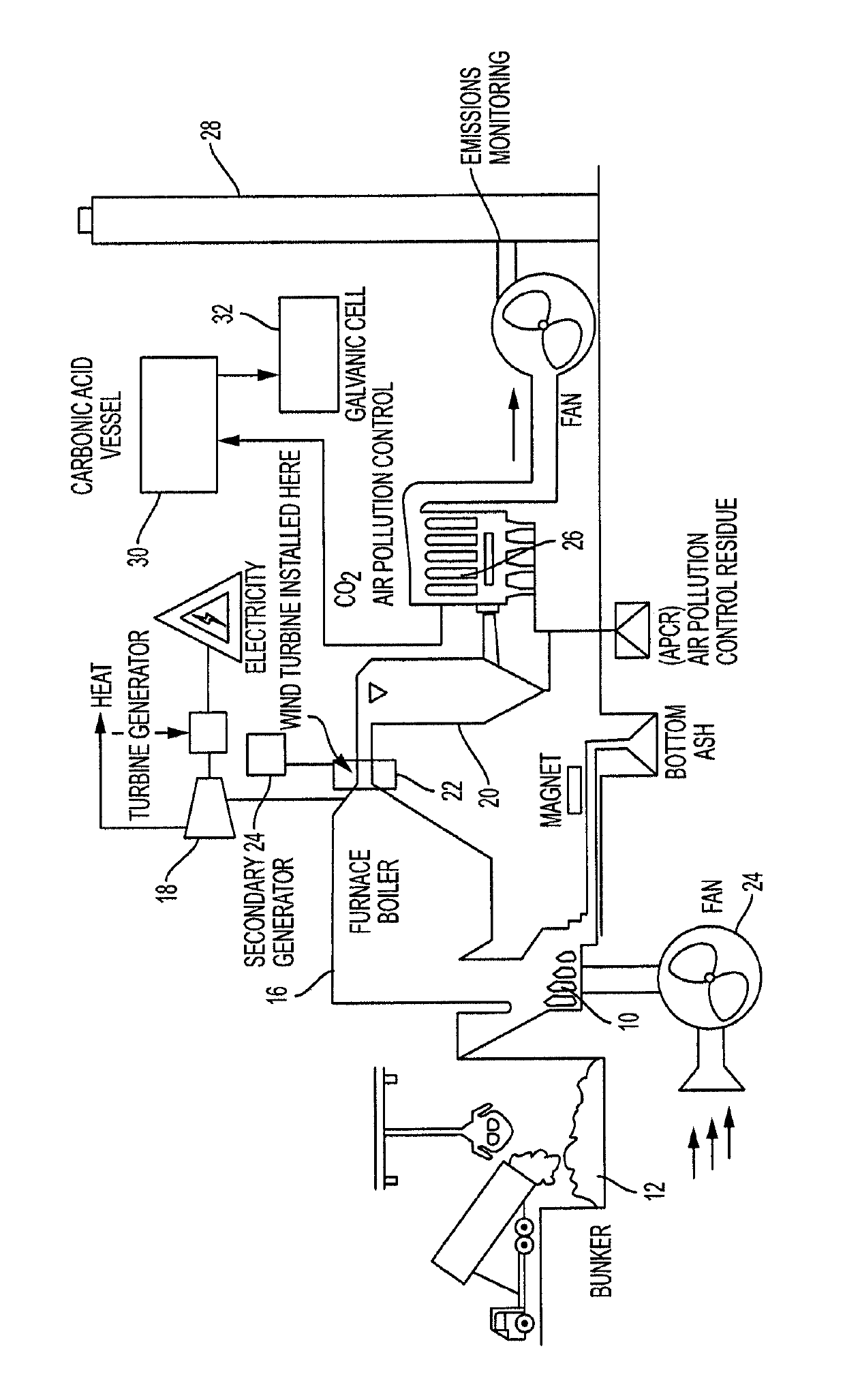
ActiveUS10483558B1High voltageIncrease capacityGas treatmentDispersed particle separationGalvanic cellKinetic energy
A system for collecting flue gas from a combustion process wherein the flue gas has elevated concentrations of carbon dioxide and converting it into electrical power and useful by-products. Kinetic energy of flue gas is used to power a wind turbine that is coupled to a generator to generate electricity. A scrubber isolates CO2 from other components of the flue gas. The CO2 is converted and stored in carbonic acid solution. The carbonic acid solution is then provided to a galvanic cell that generates electrical power and converts the reactant materials in the cell into useful by-products.
Owner:PAREHK VEDANT +1
Method for regenerating alkaline solutions
InactiveUS20170025685A1Induce precipitationFuel and primary cellsZinc oxides/hydroxidesSolventElectrolyte
This invention relates to the regeneration of spent alkaline solutions, for example, alkaline electrolyte solutions used in metal / air batteries, specifically in aluminum / air batteries. The invention provides methods and systems to regenerate alkaline electrolyte solutions by adding water and optionally other solvents to spent electrolyte solutions, thus precipitating metal hydroxides from the spent electrolyte solution.
Owner:PHINERGY
Electrolyte regeneration
ActiveUS9711804B2Reduce concentrationImprove concentrationFuel and primary cellsCellsElectrolysisPhysical chemistry
This invention is directed to electrolysis-based devices and methods for recycling of electrolyte solutions. Specifically, the invention is related to regeneration of spent electrolyte solutions comprising metal ions such as electrolyte solutions used in metal / air batteries.
Owner:PHINERGY
Regeneration System for Metal Electrodes
InactiveUS20140183047A1Low costReduce energy lossCellsFuel and primary cellsOligomerElectrical battery
The electrochemical regeneration of a replaceable metal electrode of a metal-air battery takes place in a supplementary electrochemical cell with a chemical agent oxidized on the counter electrode. The decrease of the regeneration voltage at the supplementary electrochemical cell results in the growth of the regeneration efficiency. The creation of a commercial product during chemical agent oxidation on the counter electrode decreases the overall cost of the regeneration. Possible chemical agents for regeneration include salts, metal complexes, monomers, conjugated organic molecules, oligomers or polymers.
Owner:PANISOLAR
Battery
InactiveCN1839495AFuel and primary cellsOperating means/releasing devices for valvesElectrochemical responseChemical reaction
A battery includes a battery can housing an cell that supplies electrical energy at terminals of the cell by an electro-chemical reaction with oxygen, the can including, a first member having at least one hole that is exposed to air; and a second member. The battery also includes a mechanism coupled to one of the first and second members to move the one of the first and second members such that when current is drawn from the battery, the opening in the member allows air to pass into the battery, and to move the one of the first and second members such that when current is not drawn from the battery, the opening in the member is not in registration to inhibit air to pass into the battery. The battery also includes a circuit to control the mechanism. In one embodiment the circuit monitors levels of O2 in a air plenum that surrounds the cell. The circuit to monitor levels of O2 in the air plenum includes a florescent detector / sensor that senses and responds to changes in O2 in the plenum by using the 'quenching effect' 'of oxygen on a fluorescent material.
Owner:THE GILLETTE CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com