Method for regenerating alkaline solutions
A technology of solution and mother liquor, applied in the direction of alkaline electrolyte, regeneration of useful parts, chemical instruments and methods, etc., can solve problems such as not allowing robust removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0066] Material
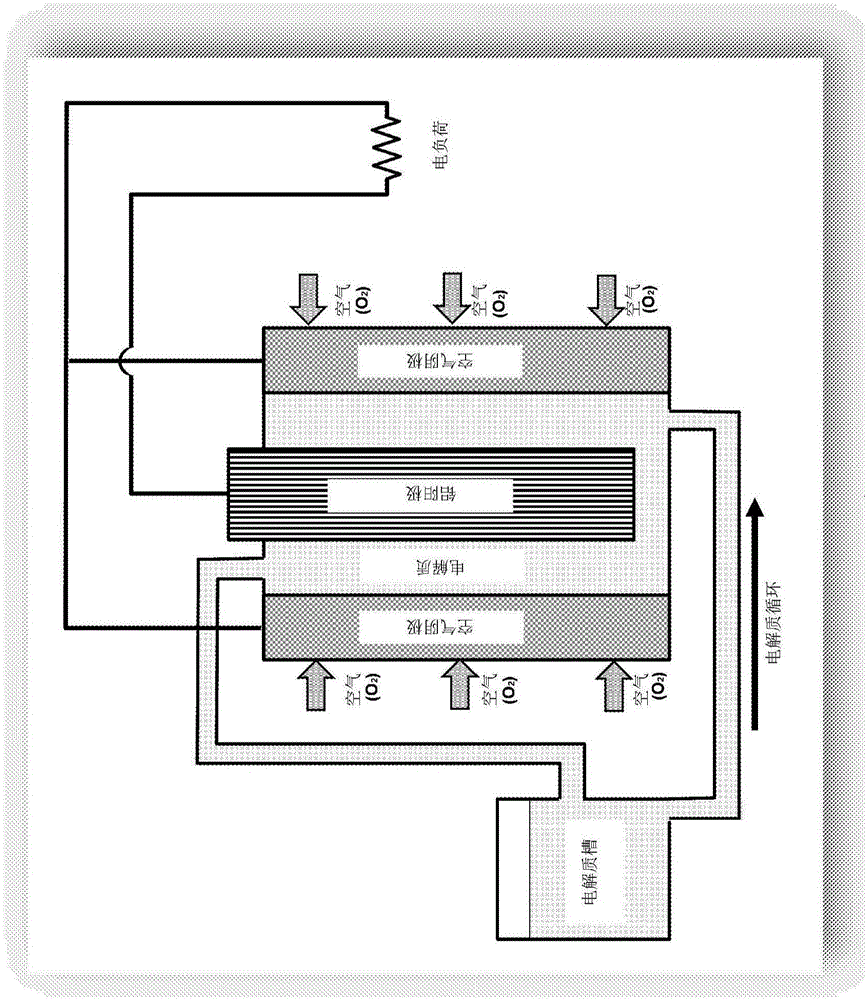
[0067] Spent electrolyte samples were obtained from aluminum-air batteries. To this end, a fresh electrolyte solution consisting of aqueous KOH (30% by weight) was allowed to circulate at a flow rate of 6 L / min through an aluminum / air battery consisting of 10 cells until K[Al(OH) 4 ] The concentration of the solution is 140-180g / liter (calculated as metal aluminum).
[0068] method
[0069] The aluminum and alkali content of the solution was titrated by a double complexing agent operation originally developed by Watts and Utley [Anal. Chem. 28, 1731 (1956)] and improved by Metrohm AG ["Determination of total caustic, total soda and aluminum in Bayer process liquors with 859 Titrotherm”, Application Note 313e, METROHM AG]. Titration analysis with the help of Metrohm Tiamo TM The Metrohm 859 Titrotherm unit operated under the software was performed.
[0070] Powder x-ray diffraction (XRD) patterns were recorded using a BRUKER D8 ADVANCE X-ray Powder Di...
example 1
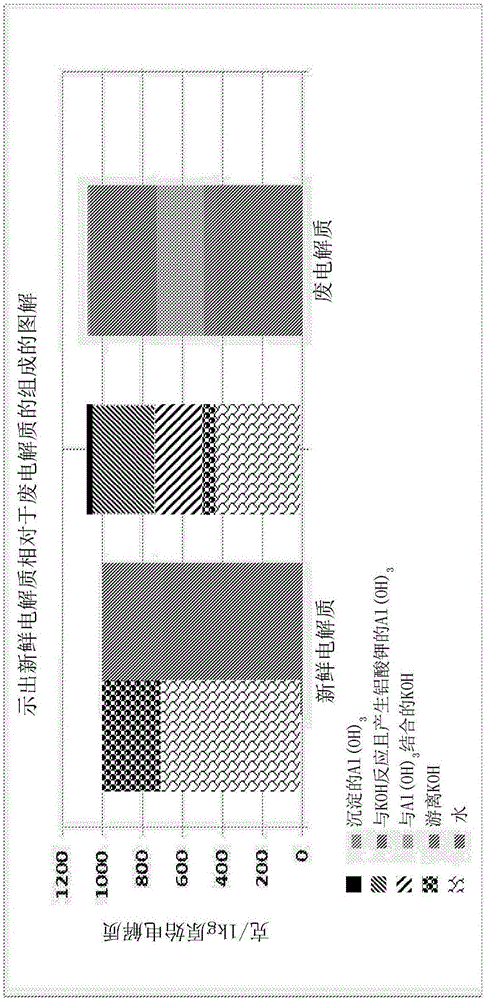
[0073] Hydrolysis of Spent Electrolyte - Liquid Phase Composition of Treated and Untreated Electrolyte
[0074] The following experiments were performed to demonstrate the effect of water addition on spent electrolyte solutions of aluminum-air batteries.
[0075] In a first experiment (comparative, untreated), 100 ml of spent electrolyte solution with 147 g / L aluminate (as Al) was added to a plastic tank. The jars were closed and allowed to stand at room temperature for a period of approximately 180 hours.
[0076] In a second experiment (water treated), 100ml of the same spent electrolyte solution (147g / L dissolved Al) was added to a plastic tank followed by water (40ml). The solution thus formed was stirred for two hours. The jars were closed and allowed to stand at room temperature for a period of approximately 180 hours.
[0077] During the 180-hour storage period, samples of both K[Al(OH) 4 ] solution and measure the concentration of aluminum dissolved in the aqueou...
example 2
[0082] Hydrolysis of Spent Electrolyte - Solid Phase Characterization
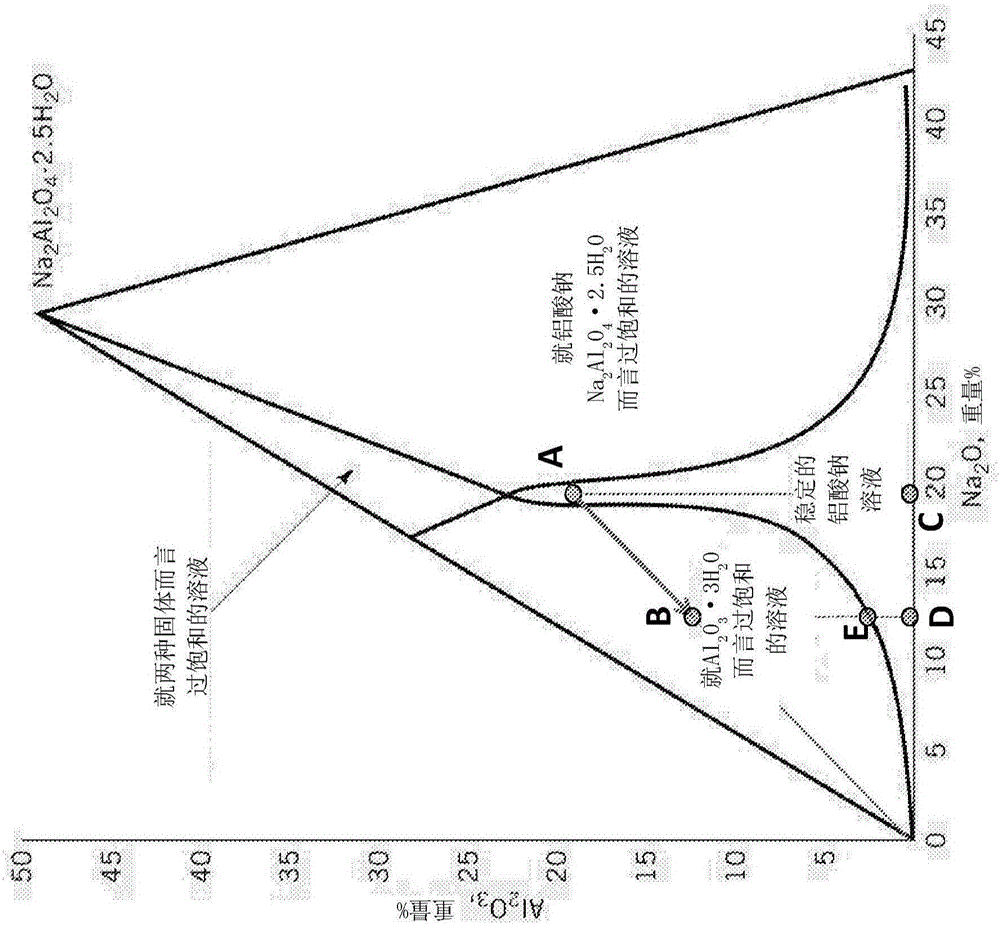
[0083] As shown in Example 1, after water addition, in K[Al(OH) 4 ] The white precipitate formed in the solution was isolated by filtration. XRD analysis indicated that the isolated solid was aluminum hydroxide in the form of gibbsite (sometimes called gibbsite). Figure 6 The X-ray powder diffraction pattern of the product is shown. The particle size distribution of the aluminum hydroxide thus formed is in the Figure 7 As described in , the indicated particle size is in the range of 1 to 10 μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com