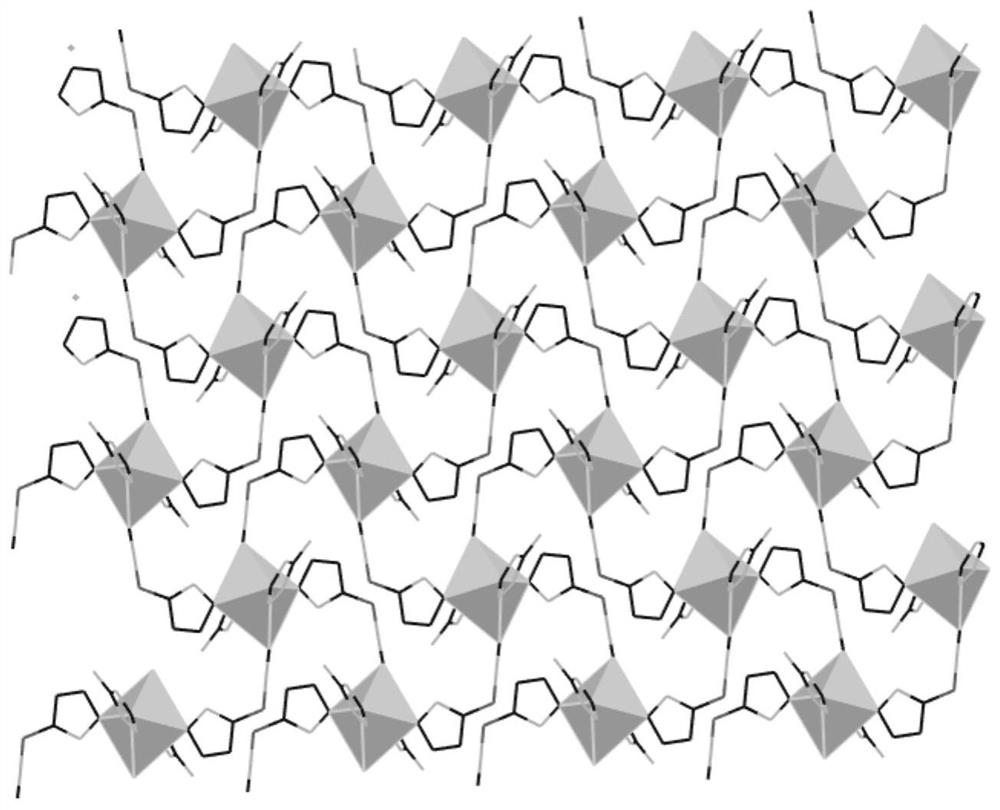
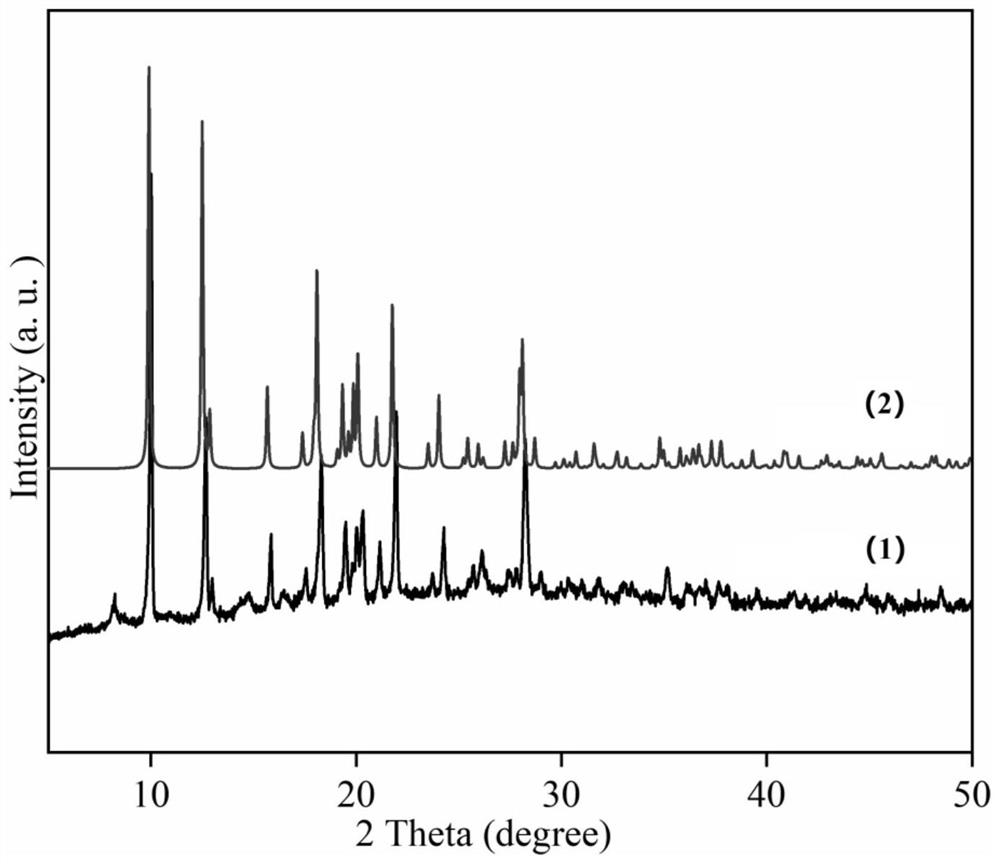
Cyano(1h-tetrazolyl)dihydroborane imidazole coordination polymer, its preparation and application
A technology of dihydroboridine imidazole and coordination polymer, which is applied in the production of offensive equipment, explosives, compressed gas, etc., can solve the problems of no solid fuel, high toxicity, strong volatility, etc., and achieve less environmental pollution and generate High enthalpy and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation method of cyano group (1H-tetrazolyl) dihydroborane 1-methyl imidazolium copper, described method step is as follows:
[0061] 0.242g (1mmol) copper nitrate trihydrate was dissolved in 5mL deionized water to obtain a copper nitrate solution;
[0062] Dissolve 0.220 g (2 mmol) of sodium cyano(1H-tetrazolyl) dihydroborane in 5 mL of deionized water to obtain a sodium cyano(1H-tetrazolyl) dihydroborane solution;
[0063] Add 0.164g (2mmol) of 1-methylimidazole to 20mL of ethanol, place it in a round bottom flask, and stir at 70°C to completely dissolve 1-methylimidazole in ethanol to obtain a 1-methylimidazole solution;
[0064] Add the copper nitrate solution dropwise to the 1-methylimidazole solution, and react at 70°C for 30 minutes to obtain a reaction solution; then add the cyano(1H-tetrazolyl)sodium dihydroborane solution dropwise to the reaction solution After the dropwise addition, continue to react at 70°C for 2 hours, cool to room temperature, an...
Embodiment 2
[0072] The preparation method of cyano group (1H-tetrazolyl) dihydroborane 1-ethyl imidazolium copper, described method step is as follows:
[0073] 0.242g (1mmol) copper nitrate trihydrate was dissolved in 5mL deionized water to obtain a copper nitrate solution;
[0074] Dissolve 0.220 g (2 mmol) of sodium cyano(1H-tetrazolyl) dihydroborane in 5 mL of deionized water to obtain a sodium cyano(1H-tetrazolyl) dihydroborane solution;
[0075] Add 0.192g (2mmol) of 1-ethylimidazole into 20mL of ethanol, place it in a round bottom flask, and stir at 70°C to completely dissolve 1-ethylimidazole in ethanol to obtain a 1-ethylimidazole solution; Add the copper nitrate solution dropwise to the 1-ethylimidazole solution, and react at 70°C for 30 minutes to obtain a reaction solution; then add the cyano(1H-tetrazolyl)sodium dihydroborane solution dropwise to the reaction solution After the dropwise addition, continue to react at 70°C for 2 hours, cool to room temperature, and filter. Th...
Embodiment 3
[0083] The preparation method of cyano group (1H-tetrazolyl) dihydroborane 1-allyl imidazolium copper, described method step is as follows:
[0084] 0.216 g (2 mmol) of 1-allylimidazole was dissolved in 20 mL of ethanol to obtain a 1-allyl imidazole solution.
[0085] Dissolve 0.220 g (2 mmol) of sodium cyano(1H-tetrazolyl) dihydroborane in 5 mL of deionized water to obtain a sodium cyano(1H-tetrazolyl) dihydroborane solution;
[0086] 0.242g (1mmol) copper nitrate trihydrate was added to 5mL deionized water, placed in a round bottom flask, stirred at 70°C, and copper nitrate trihydrate was dissolved in deionized water to obtain a copper nitrate solution; the cyano group ( Add 1H-tetrazolyl)sodium dihydroborane solution dropwise into the copper nitrate solution, react at 70°C for 30min to obtain a reaction solution; then add 1-allylimidazole solution dropwise into the reaction solution, dropwise After the addition, continue to react at 70°C for 2 hours, cool to room temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com