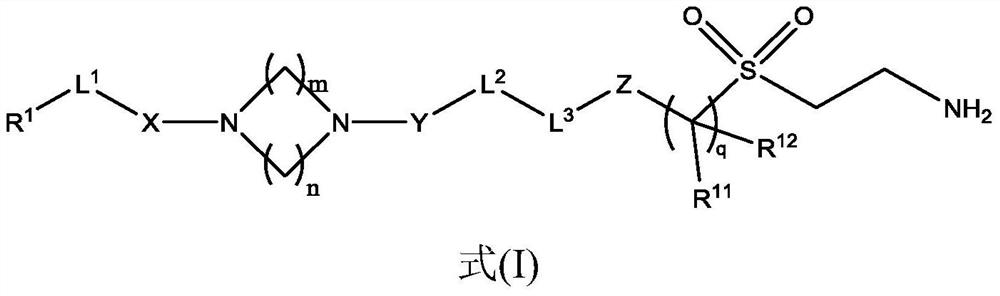
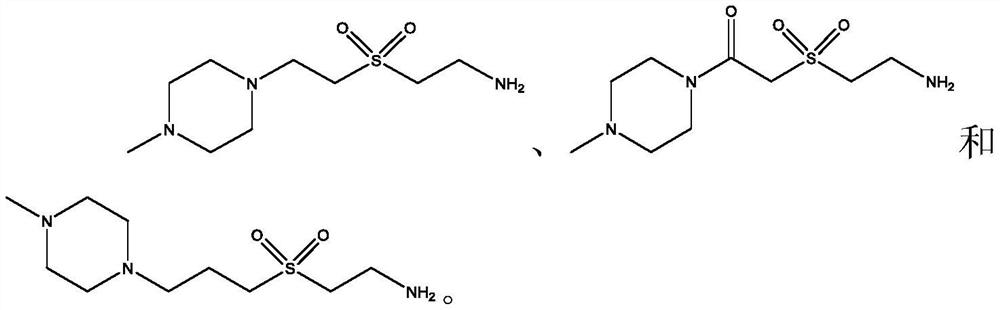
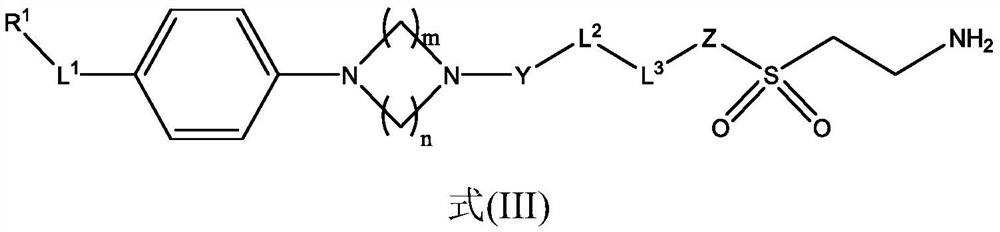
Lox inhibitors
A compound, selected technology, applied in the field of pathological compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1032] Example 1: 2-((2-((1R,5S)-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2] Nonan-3-yl)ethyl)sulfonyl)ethan-1-amine (HCl salt)
[1033]
[1034] Using GP3, with (1R,5S)-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonane (600mg, 2.19mmol ), the intermediate N-benzyl-2-((2-((1R,5S)-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazo Heterobicyclo[3.2.2]nonan-3-yl)ethyl)sulfonyl)ethan-1-amine as a colorless oil (569.2 mg, 52% yield for two steps). 1 H NMR (500MHz, DMSO-d 6 )δ7.33-7.27(m,4H),7.24-7.20(m,1H),6.78-6.74(m,2H),6.51-6.47(m,2H),3.89(q,J=7.0Hz,2H) ,3.69(s,2H),3.44-3.36(m,1H),3.33-3.21(m,5H),3.16-3.12(m,1H),3.10-3.04(m,1H),3.02-2.93(m, 2H), 2.90(t, J=6.5Hz, 2H), 2.84-2.79(m, 2H), 2.33-2.26(m, 1H), 2.21(dd, J=11.5, 6.0Hz, 2H), 1.78(d ,J=12.8Hz,1H),1.29-1.20(m,4H),1.11(s,3H),0.76(s,3H);C 28 h 42 N 3 o 3 S(M+H + ) HRMS calculated value 500.2947; measured 500.5716.
[1035] Debenzylation, purification by carbamate protection, flash chromatography (...
Embodiment 5
[1039] Example 5: 4-(4-((1R,5S)-3-(2-((2-aminoethyl)sulfonyl)ethyl)-9,9-dimethyl-3,6-diazo Heterobicyclo[3.2.2]nonan-6-yl)phenyl)thiomorpholine 1,1-dioxide (HCl salt)
[1040]
[1041] Using GP4 steps 1-3 with 2-bromoethanol (1.80 mL, 25.4 mmol), the intermediate tert-butyl (2-((2-bromoethyl)sulfonyl)ethyl)carbamate was obtained as Yellow solid (1.15 g, 38% yield over three steps). 1H NMR (500MHz, CDCl3) δ5.16(br s, 1H), 3.72-3.64(m, 4H), 3.55(dd, J=8.4 and 6.9Hz, 2H), 3.32(dd, J=6.9 and 5.1Hz ,2H), 1.46(s,9H).
[1042] Using GP4 steps 4-5 with 4-(4-((1R,5S)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonan-6-yl)phenyl ) thiomorpholine 1,1-dioxide (130mg, 0.36mmol), obtained 4-(4-((1R,5S)-3-(2-((2-aminoethyl)sulfonyl)ethyl base)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonan-6-yl)phenyl)thiomorpholine-1,1-dioxide (HCl salt) (49.3 mg, 23% yield over two steps). 1H NMR (500MHz, DMSO-d6)δ; C23H39N4O 4 HRMS calculated value of S2 (M+H+) 499.2413; found 499.2418.
Embodiment 6
[1043] Example 6: 2-((3-((1R,5S)-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2] Nonan-3-yl)propyl)sulfonyl)ethan-1-amine (HCl salt)
[1044]
[1045] Using GP4 steps 1-3 with 3-bromopropanol (2.71 mL, 30 mmol), the intermediate tert-butyl (2-((3-bromopropyl)sulfonyl)ethyl)carbamate was obtained as yellow Solid (2.65 g, 27% over three steps). 1H NMR (500MHz, CDCl 3 )δ5.17(br s,1H),3.67(q,J=6.0Hz,2H),3.56(t,J=6.2Hz,2H),3.25(t,J=6.0Hz,2H),3.23-3.19 (m,2H), 2.46-2.40(m,2H), 1.46(s,9H).
[1046] Using GP4 steps 4-5 with (1R,5S)-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonane ( 83.1 mg, 0.30 mmol), 2-((3-((1R,5S)-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo [3.2.2] Nonan-3-yl)propyl)sulfonyl)ethane-1-amine (HCl salt) (90.1 mg, 98% yield in two steps). 1 H NMR (500MHz, DMSO-d 6 )δ8.49,8.39(2br s,2H),6.85-6.79(m,2H),6.76-6.71(m,1H),6.65-6.61(m,1H),3.91(2q,J=7.0Hz,2H ),3.80-2.98(m,15H),2.60-2.47(m,1H),2.38-2.04(m,2H),1.76(d,J=14.4Hz,1H),1.64,1.45(2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com