Polyimide/cotton fiber hydrogen peroxide synthesis photocatalyst as well as preparation and application thereof
A technology of cotton fiber and polyimide, applied in the field of continuous synthesis of hydrogen peroxide, can solve unreached problems and achieve the effects of easy control, good processability, green safety and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Stir and dissolve polyamic acid monomer diamine and dianhydride with a molar ratio of 1:1 (0.01mol:0.01mol) successively in 120mL N,N-dimethylformamide, add a few drops of isoquinoline, and stir to react The polyamic acid solution was formed overnight, and then the cotton fiber (5g) with a clean surface was placed in it, and mechanically stirred evenly, and then the reaction system was placed under the protection of a nitrogen flow, and the reaction temperature range was controlled at 120°C for 16 hours. Natural cooling, Filter, wash with ethanol and water to obtain a polyimide / cotton fiber hydrogen peroxide synthesis photocatalyst, and the polyimide / cotton loading capacity is 0.6 g / g.
[0050] In this example, polyamic acid monomer (diamine is 1,4-phenylenediamine, dianhydride is benzene-1,2,4,5-tetracarboxylic dianhydride) forms polyamic acid, and then ring-closing polycondensation forms The reaction formula of polyimide is as follows:
[0051]
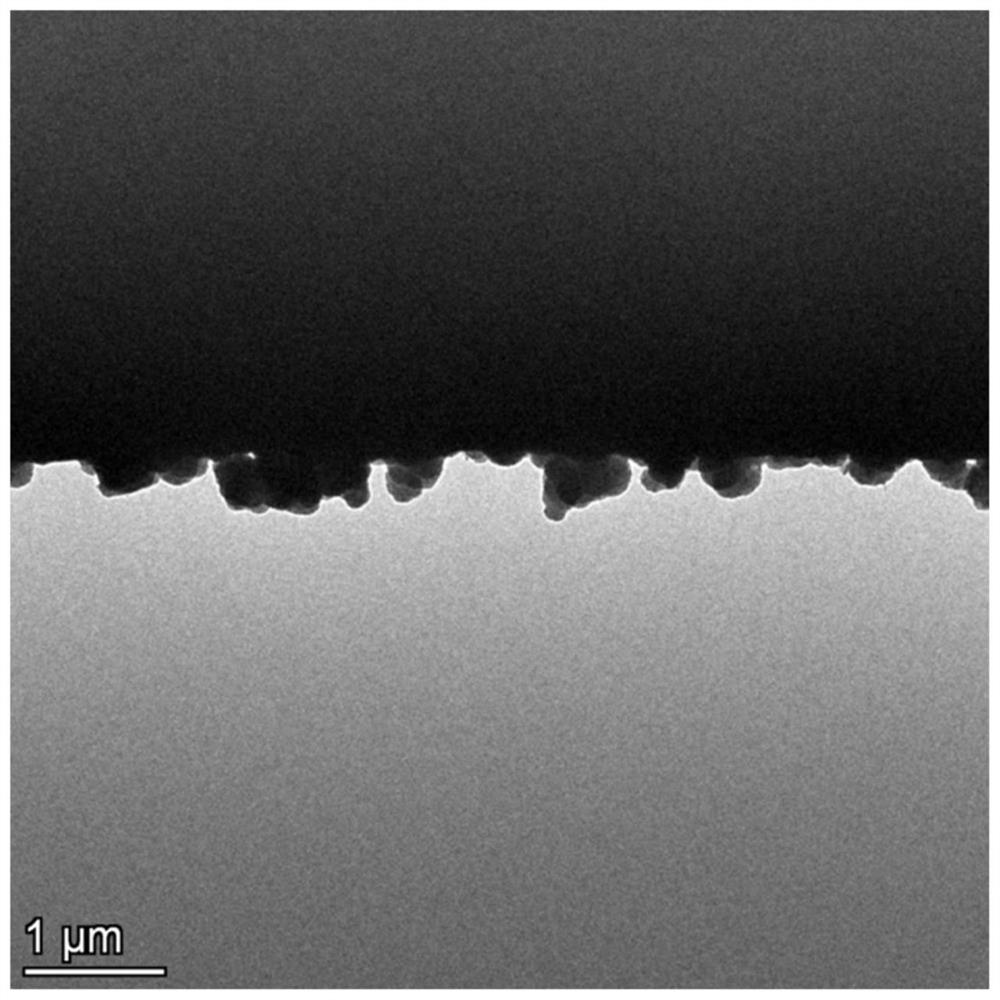
[0052] figure 1 ...
Embodiment 2
[0059] Dissolve polyamic acid monomer diamine and dianhydride with a molar ratio of 1:1 (0.01mol:0.01mol) in 120mL dimethyl sulfoxide successively, add a few drops of isoquinoline, and stir overnight to form polyamide acid solution, and finally put the cotton fiber (5g) with clean surface in it, mechanically stir it evenly, and finally put the reaction system under the protection of nitrogen flow, control the reaction temperature range 180 ℃, time 8 hours, natural cooling, filtration, ethanol and The polyimide / cotton fiber hydrogen peroxide synthesis photocatalyst was obtained by washing with water, and the loading capacity of the polyimide / cotton was 0.6 g / g.
[0060] Put 20g of the above photocatalyst in the reactor, fill it up with water, adjust the flow rate of the gravity dropper to 100mL / h, and produce a stable hydrogen peroxide solution with a concentration of about 79mg / L after 8 hours of sunlight.
Embodiment 3
[0062] The polyamic acid monomer diamine and dianhydride with a molar ratio of 1:1 (0.005mol:0.005mol) were successively stirred and dissolved in 120mL of m-cresol, and a few drops of isoquinoline were added, and the reaction was stirred overnight to form polyamic acid solution, and finally put the cotton fiber (5g) with clean surface in it, mechanically stir it evenly, and then put the reaction system under the protection of nitrogen flow, control the reaction temperature range of 190°C for 16 hours, cool naturally, filter, ethanol and water The polyimide / cotton fiber hydrogen peroxide synthesis photocatalyst was obtained by washing, and the loading capacity of the polyimide / cotton was 0.3 g / g.
[0063] Put 20g of the above-mentioned photocatalyst in the reactor, fill it up with water, adjust the flow rate of the gravity dropper to 100mL / h, and produce a stable hydrogen peroxide solution with a concentration of about 65mg / L after 8 hours of sunlight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com