3D-printed tumor vaccine composition as well as preparation method and application thereof
A tumor vaccine, 3D printing technology, applied in the direction of anti-tumor drugs, drug combinations, medical preparations of non-active ingredients, etc., can solve the problems of difficult batch and stable manufacturing and storage, toxic and side effects, etc., to promote identification and intake , low side effects, small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of 3D scaffold immune vaccine printing ink and construction of scaffold vaccine
[0054] Weigh sodium alginate and dissolve it in 10mL deionized water, and fully mix and dissolve under the mixer. After obtaining a uniform sodium alginate solution, weigh a certain amount of gelatin and add it to the sodium alginate solution, and place it in an oil bath at 60° C. for 30 minutes to fully dissolve the gelatin to obtain a printable ink. Weigh the tumor-associated antigen, dissolve it in PBS, add the prepared sterile antigen solution into the printing ink, and mix the antigen solution and the printing ink fully through physical mixing. The prepared tumor vaccine printing ink was sonicated for 1 minute, and then centrifuged at 1500-3000 rpm for 3 minutes to remove air bubbles in the solution, so as to avoid affecting the stability of subsequent printing and reducing the mechanical properties of the material. After removing air bubbles, draw the prepared...
Embodiment 2
[0056] Example 2: Relevant characterization of 3D printed scaffold vaccines
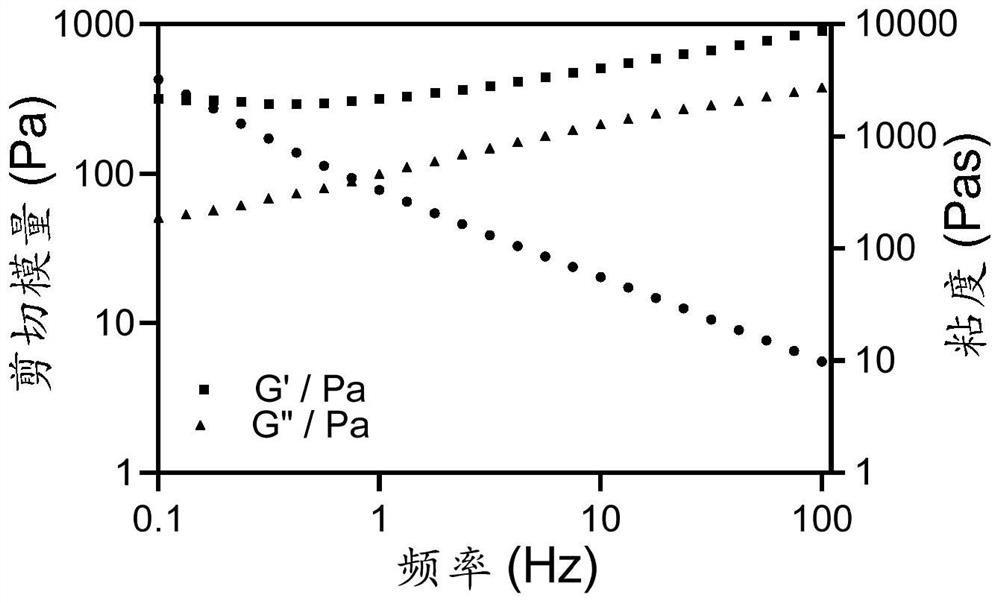
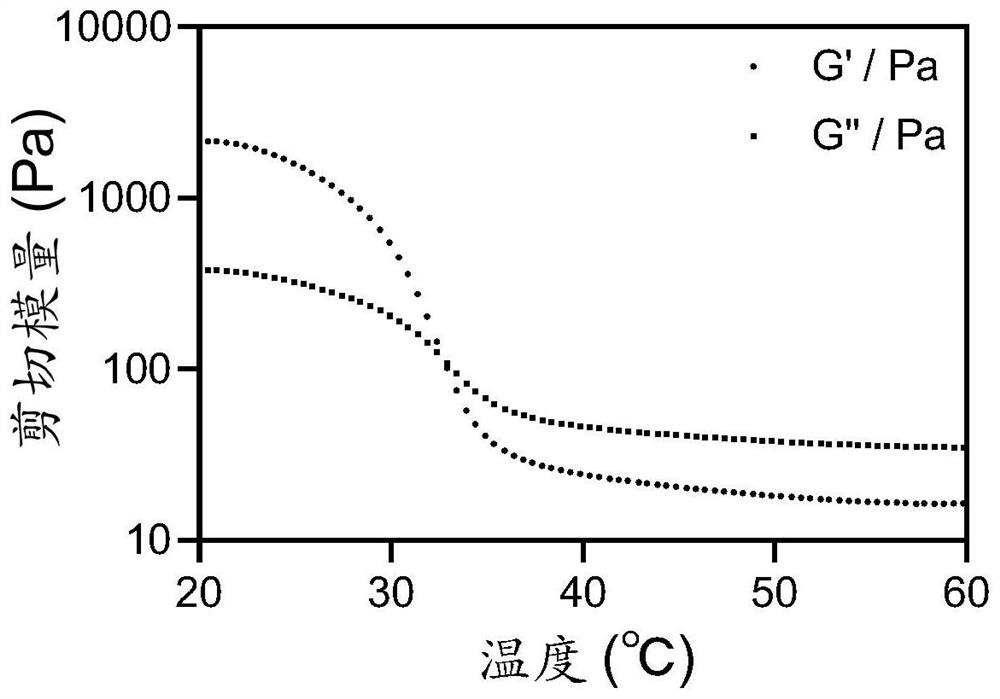
[0057] First, we verified the relevant rheological properties of tumor vaccines through rheological experiments. The results are as follows: figure 2 , image 3 As shown, with the change of shear frequency, the tumor vaccine printing ink still has good rheological properties. Furthermore, a gel-sol transition occurs with increasing temperature. XRD analysis proves that before and after adding the antigen, the material has an amorphous structure, such as Figure 4 shown. A mechanical testing system was used to study the mechanical properties of the printing ink before and after adding the antigen. The results are as follows: Figure 5 As shown, after adding the antigen, the mechanical properties of the material are improved to a certain extent, and its tensile stress, breaking strain and compressive stress are all effectively improved, which proves that the antigen can act as a component in the s...
Embodiment 3
[0058] Example 3: Biosafety Analysis of 3D Printed Preparation Vaccines
[0059] Mice were subcutaneously inoculated with scaffold vaccine, and the in vivo degradation of scaffold vaccine was studied. The results are as follows: Figure 8 As shown, with the prolongation of implantation time, the stent vaccine gradually degraded, and on the seventh day, most of the stent vaccine was degraded. In addition, the scanning electron microscope pictures also studied the morphology and structure of the scaffold vaccine at different time points, which also proved the degradation of the scaffold vaccine in vivo. At the same time, the skin tissue in the vaccinated area was collected to study the toxic and side effects of the relevant area after vaccination. It can be found through the HE stained section of the skin tissue that there is no obvious damage to the skin in the vaccinated area, which proves that the scaffold vaccine has good Biosafety and low side effects, the experimental res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com