Aav gene therapy for treating nephrotic syndrome
A nephrotic syndrome, gene therapy technology, applied in the field of treatment of monogenic forms of nephrotic syndrome, can solve the problem of not exploring AAV serotypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0052] method
[0053] Vector production
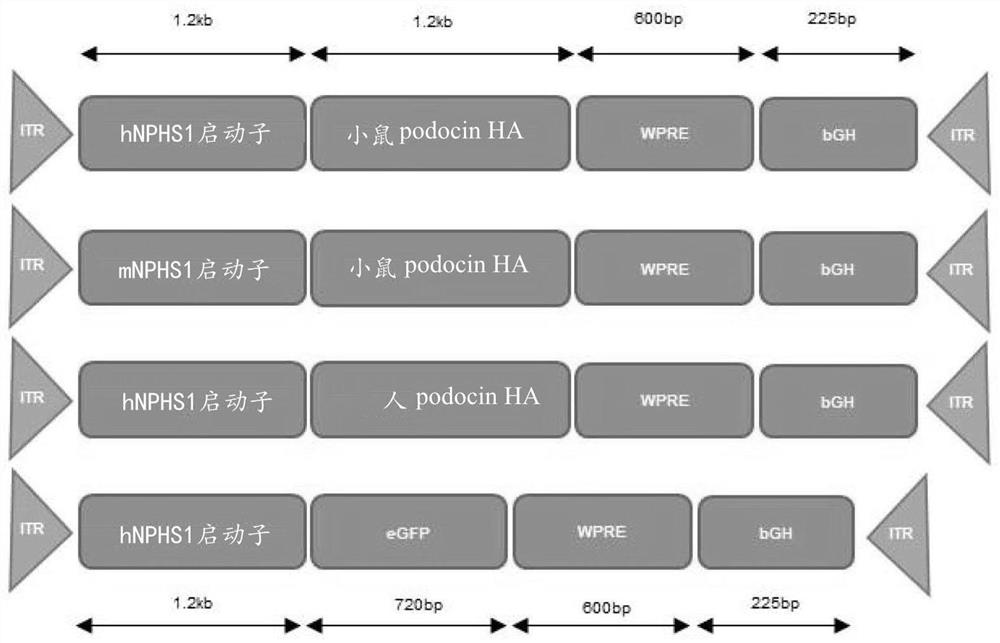
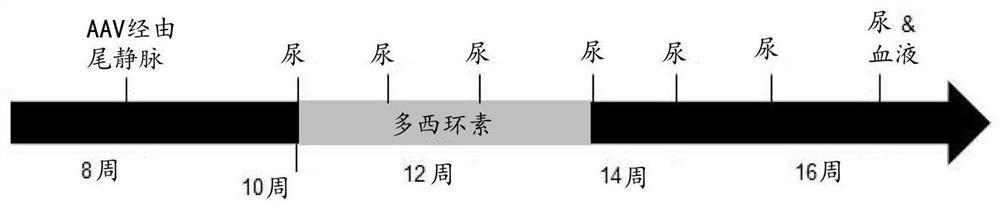
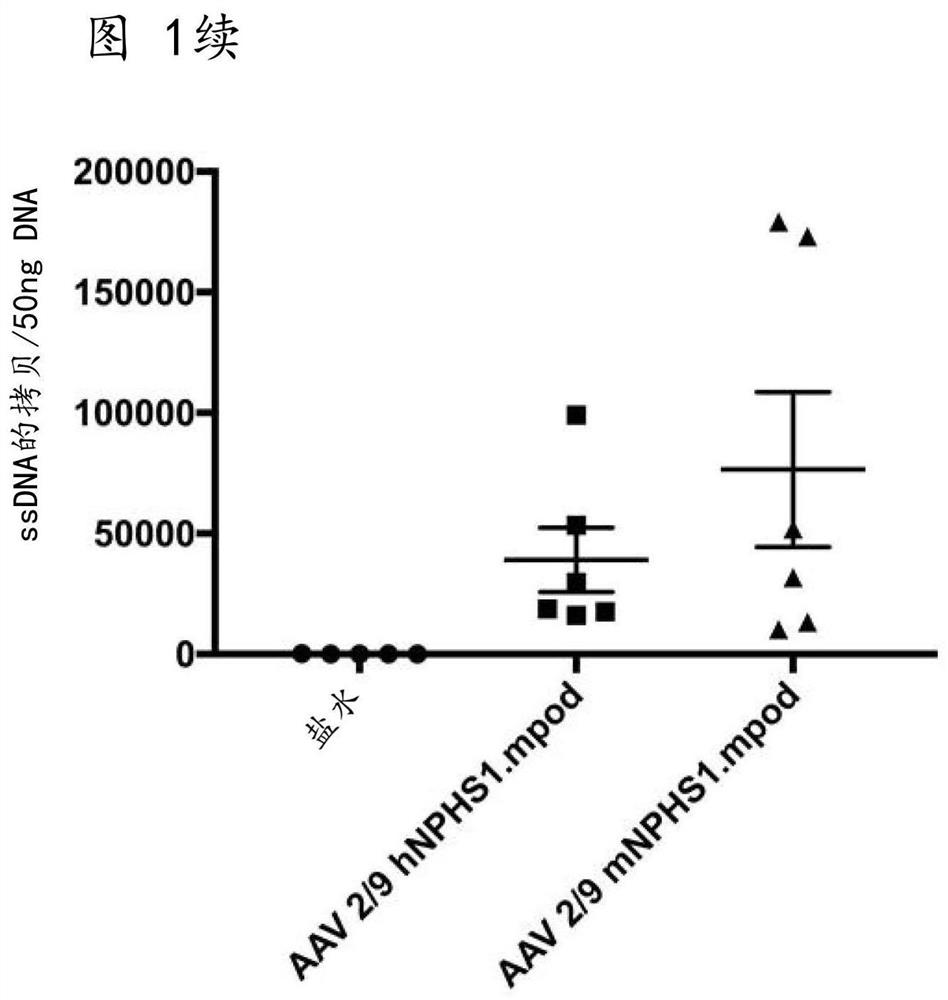
[0054] We use human ( Figure 6) and mouse (sequence not shown) podocin cDNA (Origene, Herford, Germany) and human VDR and Smad7 cDNA, pAV.hNPHS1.mpodHA.WPRE was prepared from the CMV eGFP L22Y pUC-AV2 construct (a kind gift of Amit Nathwani) .bGH, pAV.mNPHS1.mpodHA.WPRE.bGH and pAV.hNPHS1.hpodHA.WPRE.bGH ( Figure 1A ) pAV.mNPHS1.hHAVDR.WPRE.bGH and pAV.mNPHS1.hHASmad7.WPRE.bGH. Human embryonic kidney 293T cells were transfected with capsid plasmids (pAAV9 from Penn Vector Core, pAAV LK03 was a kind gift from Mark Kay), helper plasmids with adenovirus genes, and transgenic plasmids using polyethyleneimine. Cells and supernatants were harvested 72 hours after transfection. Cells were subjected to 5 freeze-thaw cycles, while supernatants were subjected to PEG precipitation (8% PEG 0.5N NaCl). These were combined and incubated with 0.25% sodium deoxycholate and 70 units / ml Benzonase at 37°C for 30 minutes. Vector was purified by io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com