A kind of para-position c-h alkylated aromatic amine and preparation method thereof
A technology for alkylating arylamine and C-H, which is applied in the field of alkylating arylamine and its preparation, can solve the problems of harsh reaction conditions, high reaction temperature, and can not use drug para-reaction, and achieves mild reaction conditions and environmental friendliness. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
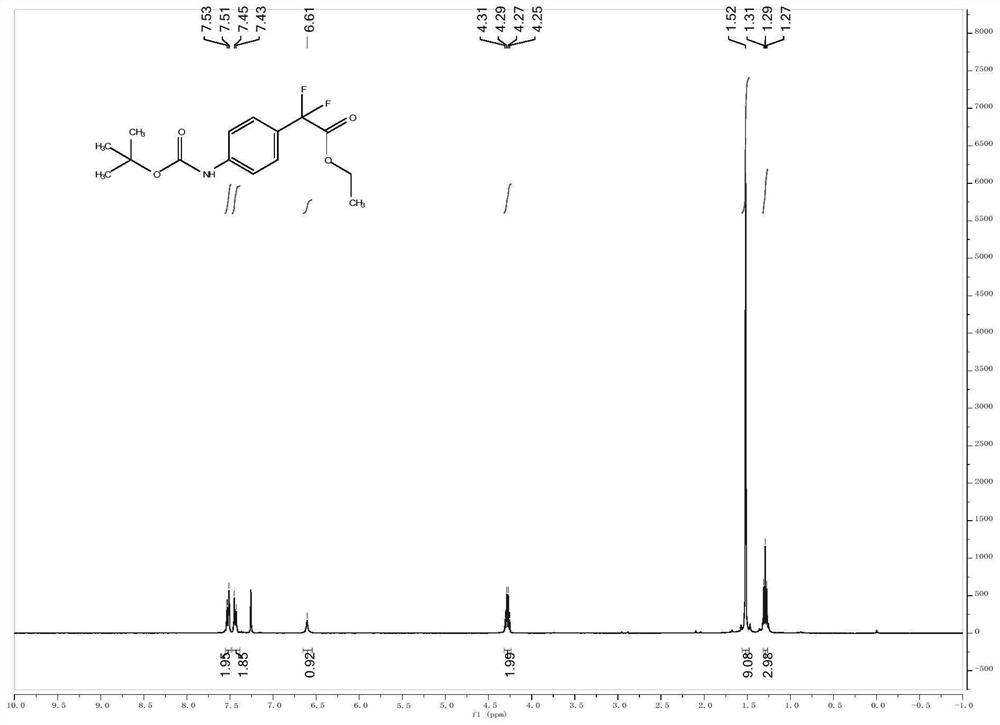
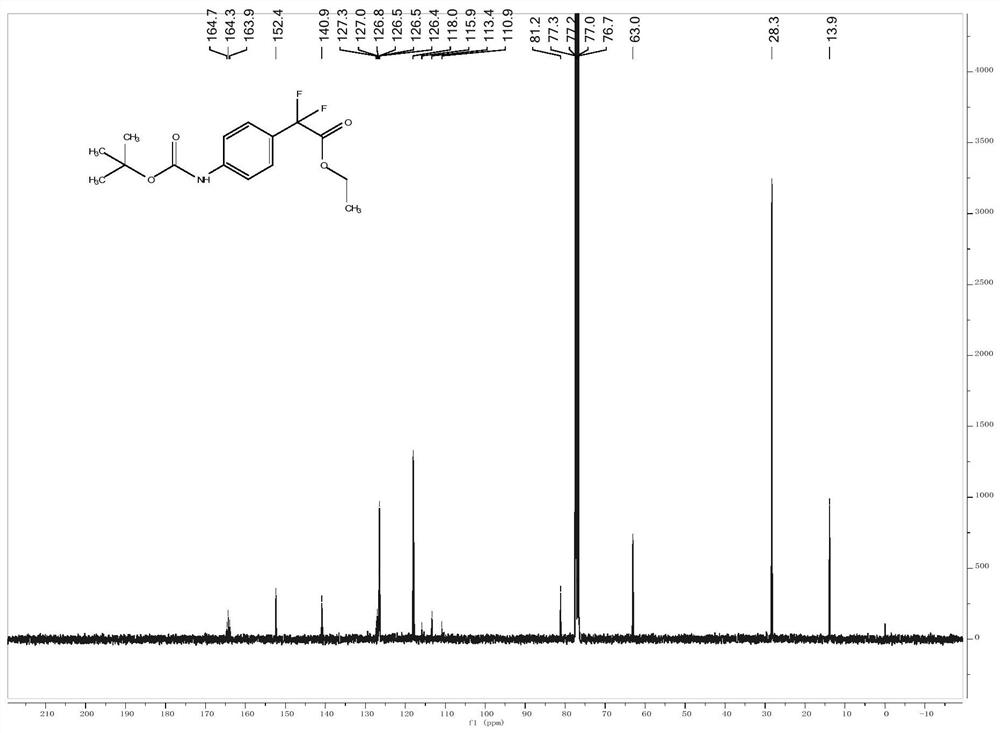
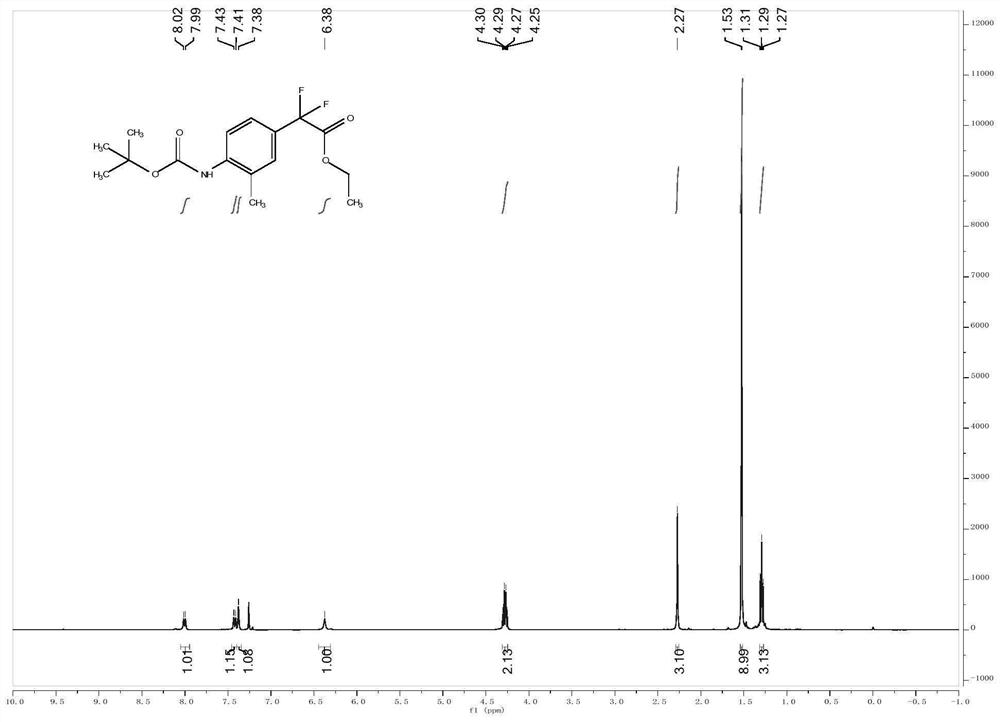
[0027] Embodiment 1: The structural formula of a para-position C-H alkylated arylamine of the present embodiment is: Wherein A is C, O, P or S; R 1 , R 2 and R 3 is a fluorine-containing or fluorine-free organic group, and the organic group is an alkyl group, an alkenyl group, an alkynyl group, a nitro group, a cyano group or an aryl group; the value range of n is 0
specific Embodiment approach 2
[0028] Specific embodiment two: This embodiment is a kind of preparation method of para-position C-H alkylated arylamine is completed according to the following steps:
[0029] Add aromatic amine, derivative, catalyst, phosphorus-containing ligand, alkali, silver salt and solvent to the Schlenk tube, mix well, and then react at a temperature of 0 ° C ~ 80 ° C and light source irradiation to obtain para-position C-H alkane alkylated arylamines;
[0030] The structural formula of described aromatic amine is where A is C, O, P or S; R 1 and R 2 is a fluorine-containing or fluorine-free organic group, and the organic group is an alkyl group, an alkenyl group, an alkynyl group, a nitro group, a cyano group or an aryl group; the value range of n is 0
[0031] The structural formula of the derivative is R 3 -X, where R 3 is a fluorine-containing or non-fluorine-containing organic group, the organic group is an alkyl group, an alkenyl group, an alkynyl group, a nitro group,...
specific Embodiment approach 3
[0038] Embodiment 3: This embodiment is different from Embodiment 1 or Embodiment 2 in that the reaction time is 1 h to 102 h. Other steps are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com