Medicinal composition having excellent absorption of drug into living body and excellent chemical stability
A technology for pharmaceutical compositions and hydrates, applied in the directions of pharmaceutical combinations, active ingredients of heterocyclic compounds, pharmaceutical formulations, etc., can solve the method of chemical stability without any enlightenment, deterioration of chemical stability, and no description of deterioration of chemical stability. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
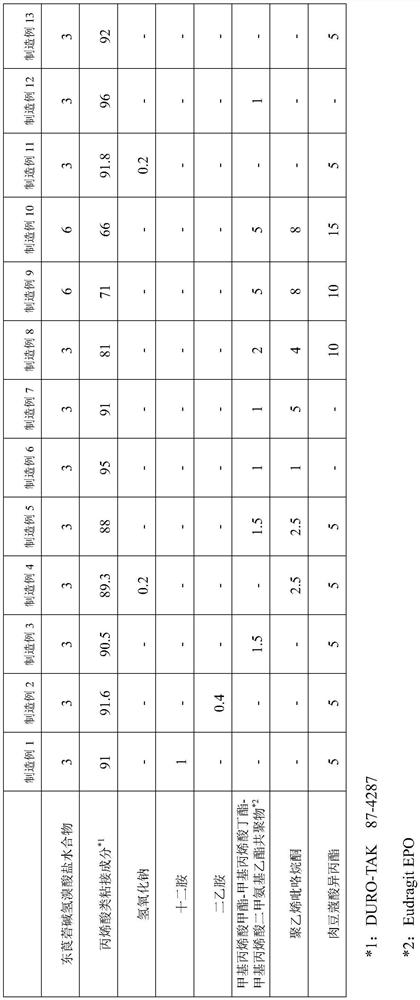
manufacture example 1
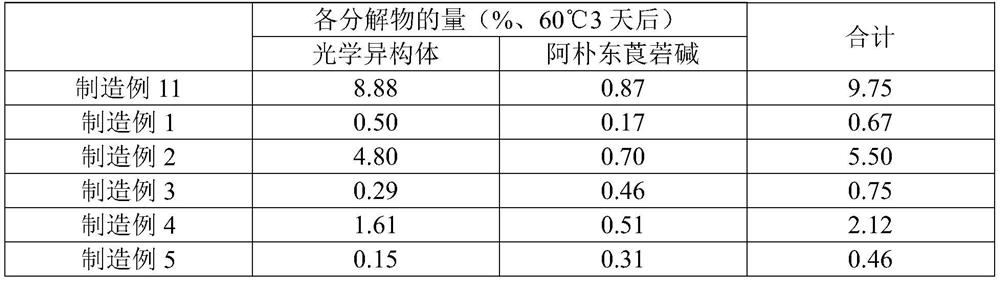
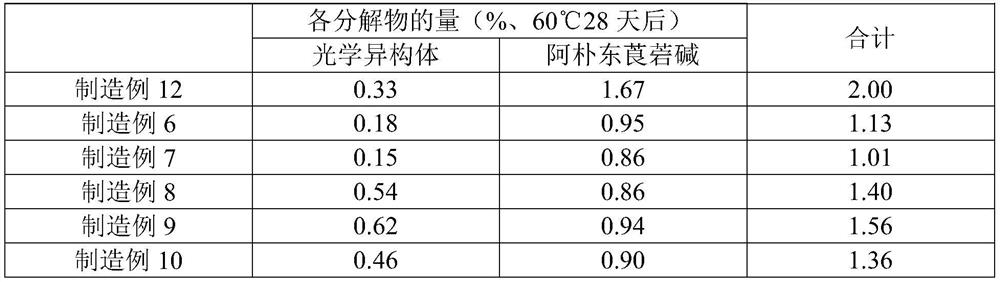
[0053] According to the formulation ratio recorded in Table 1, scopolamine hydrobromide hydrate, isopropyl myristate, and dodecylamine were dissolved in ethyl acetate / methanol mixture, and an acrylic adhesive component (trade name: DURO - TAK87-4287, produced by Henkel) and mixed and stirred to obtain a homogeneous dissolved product. Next, the dissolved product was stretched into a release film using a knife coater so that the thickness after drying became 100 μm, dried to form a drug-containing adhesive layer, and then bonded to a support. Thereafter, it is cut to a desired size to obtain a patch.
manufacture example 2
[0055] A patch was obtained in the same manner as in Production Example 1, except that diethylamine was prepared in place of dodecylamine so that the amount of scopolamine free base was equal to that in Production Example 1 according to the compounding ratio described in Table 1.
manufacture example 3
[0057] According to the formulation ratio recorded in Table 1, except that the amount of scopolamine free base is equal to that of Production Example 1, methyl methacrylate-butyl methacrylate-dimethylaminoethyl methacrylate copolymer (commercial product Name: Eudragit EPO (manufactured by Evonik)) instead of laurylamine, a patch was obtained in the same manner as in Production Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com