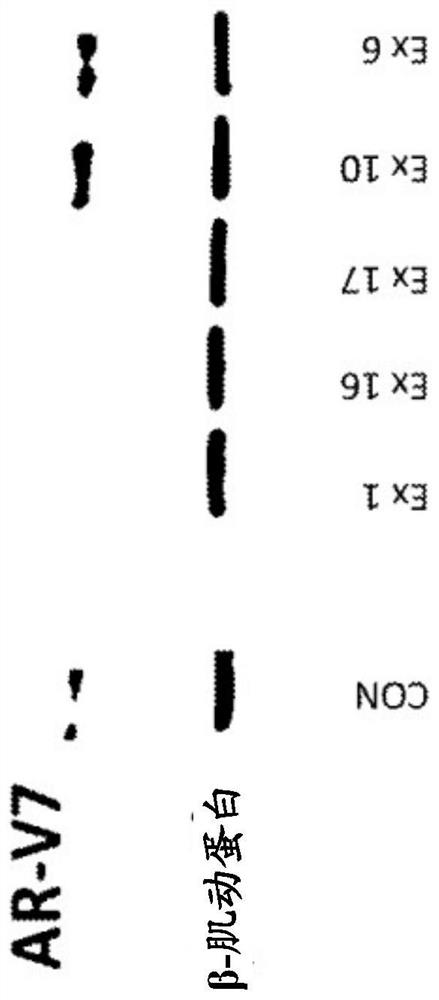
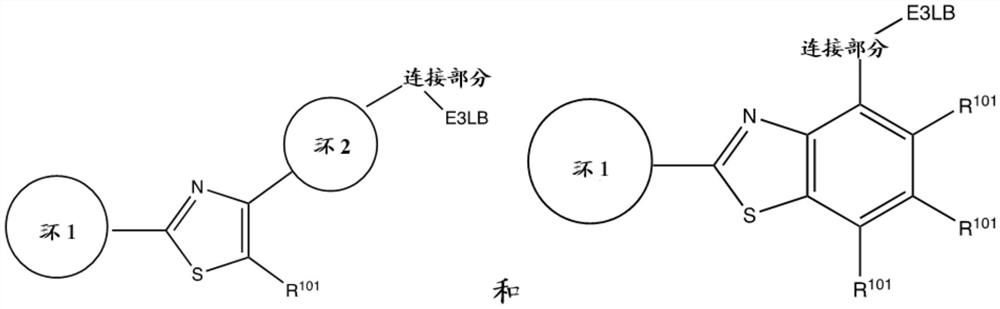
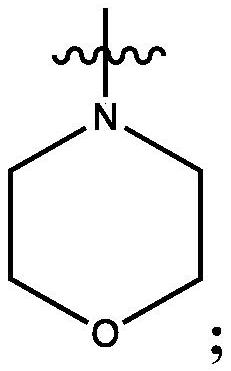
Bi-functional compounds and methods for targeted ubiquitination of androgen receptor
A compound and halogen technology, applied in organic chemistry, pharmaceutical formulation, drug combination, etc., can solve the problems of short overall survival, short PSA progression-free survival, low PSA response rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0174] By using 2-(2,3-difluoro-6-(2-morpholinothiazol-4-yl)phenoxy)acetic acid (E) (0.02 g, 0.056 mmol) in dry DMF (1.0 mL) , 4-((8-aminooctyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride (M)( 0.024g, 0.056mmol), DIPEA (0.03mL, 0.224mmol) and HATU (0.027g, 0.70mmol) for general method III through SiO 2 After purification by flash column chromatography (DCM / MeOH, 99:1-96:4), followed by further purification by HPLC (Agilent Technologies 1200; column, Eclipse XDB-C18 4.6x150 mm (5 μm); flow rate, 1.0 mL / min; DAD 190-650nm; isocratic eluent, ACN / H 2 (070:30) afforded the title compound as a yellow solid (0.016 g, 50% yield). 1 H NMR (400MHz, CDCl 3 ):δ8.14(s,1H),7.64-7.55(m,1H),7.55-7.45(m,1H),7.11(d,J=7.1Hz,1H),7.04(s,1H),7.04- 6.97(m,1H),6.93(s,1H),6.90(d,J=8.6Hz,1H),6.25(s,1H),5.02-4.86(m,1H),4.60(s,2H),3.97 -3.76(m,4H),3.64-3.48(m,4H),3.34(q,J=6.8Hz,2H),3.28(q,J=6.6Hz,2H),2.99-2.67(m,3H), 2.21-2.08(m,1H),1.81-1.31(m,12H); 13 C NMR (101MHz, CDC...
Embodiment 3
[0176] By using 2-(2,3-difluoro-6-(2-morpholinothiazol-4-yl)phenoxy)acetic acid (E) (0.04 g, 0.086 mmol) in dry DMF (2.0 mL) , 4-((10-aminodecyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride (N)( 0.04g, 0.086mmol), DIPEA (0.05mL, 0.44mmol) and HATU (0.041g, 0.107mmol) for general method III through SiO 2 Purification by flash column chromatography (DCM / acetone, 95:5-85:15) afforded the title compound as a yellow solid (0.026 g, 39% yield). 1 H NMR (400MHz, CDCl 3 ):δ8.16(s,1H),7.61(ddd,J=8.4,6.0,2.1Hz,1H),7.55-7.47(m,1H),7.10(d,J=7.1Hz,1H),7.06- 6.95(m,2H),6.94(s,1H),6.90(d,J=8.5Hz,1H),6.25(t,J=5.6Hz,1H),4.93(dd,J=12.1,5.3Hz,1H ),4.59(s,2H),3.93-3.78(m,4H),3.59-3.50(m,4H),3.38-3.23(m,4H),2.97-2.69(m,3H),2.20-2.08(m ,1H),1.74-1.64(m,2H),1.59-1.48(m,2H),1.47-1.39(m,2H),1.37-1.29(m,10H); 13 C NMR (101MHz, CDCl 3 ): δ170.97, 170.79, 169.51, 168.32, 167.89, 167.65, 150.71(dd, J=251.9, 10.9Hz), 147.04, 146.51-146.34(m), 144.21(dd, J=247.4, 14.0Hz), 144....
Embodiment 5
[0180] By using 2-(2,3-difluoro-6-(2-morpholinothiazol-4-yl)phenoxy)acetic acid (E) (0.027 g, 0.076 mmol) in dry DMF (1.0 mL) , 4-((2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethyl)amino)-2-(2,6-dioxopiperidine-3- base) isoindoline-1,3-dione hydrochloride (P) (0.037g, 0.076mmol), DIPEA (0.053mL, 0.305mmol) and HATU (0.036g, 0.095mmol) for general method III, through SiO 2 After purification by flash column chromatography (DCM / MeOH, 98:2), the title compound was obtained as a yellow solid (0.041 g, 69% yield). 1 H NMR (400MHz, CDCl 3 ):δ8.31(s,1H),7.66(t,J=6.6Hz,1H),7.50(t,J=7.8Hz,1H),7.12(d,J=7.1Hz,1H),7.09-6.96 (m,3H),6.91(d,J=8.5Hz,1H),6.48(t,J=4.8Hz,1H),4.92(dd,J=5.3,11.9Hz,1H),4.56(s,2H) ,3.97-3.81(m,6H),3.81-3.22(m,18H),3.07-2.58(m,3H),2.16-2.06(m,1H); 13 C NMR (101MHz, CDCl 3 ): δ171.00, 170.63, 169.24, 168.30, 168.05, 167.58, 150.54 (dd, J = 11.4, 251.2 Hz), 146.79, 146.23-145.77 (m), 144.52 (d, J = 9.3 Hz), 144.29 (dd, J =13.9,247.2Hz),136.02,132.51,125.28,124.43,116....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com