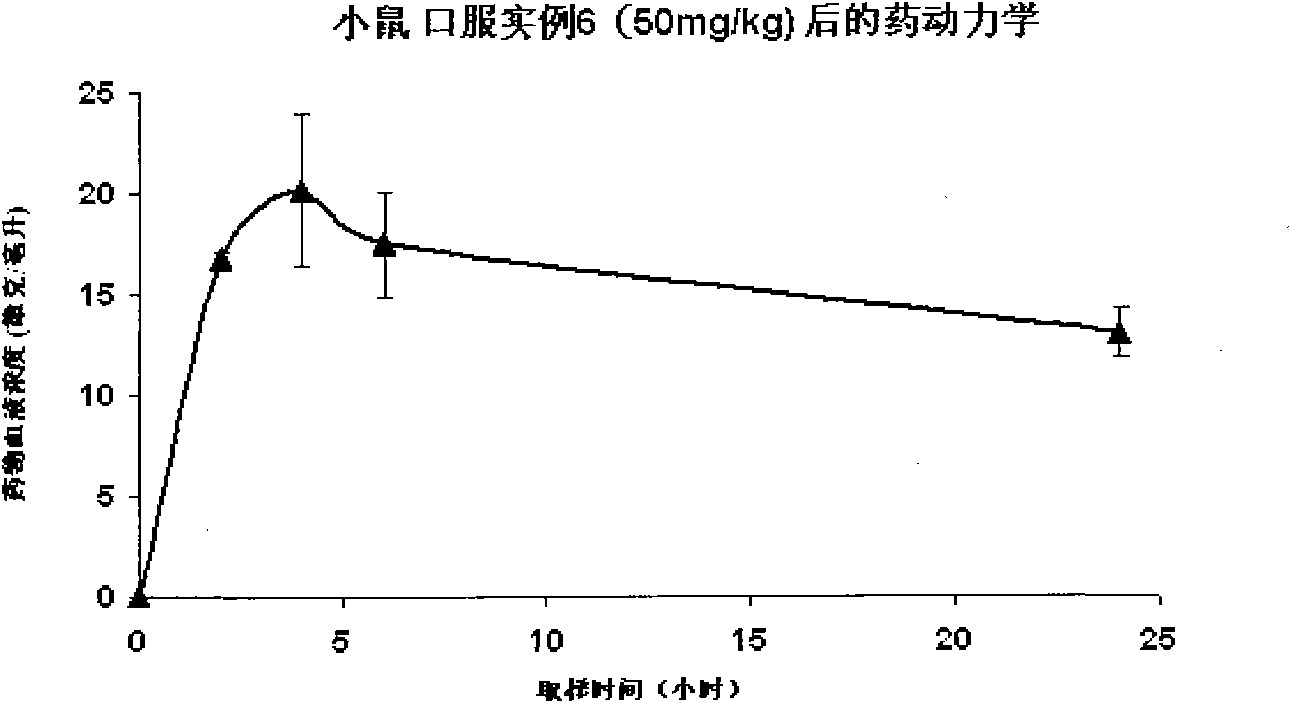
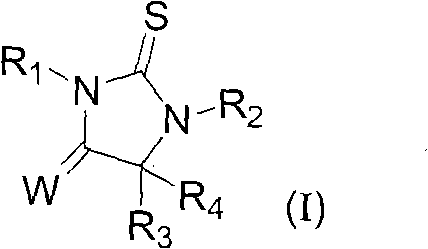
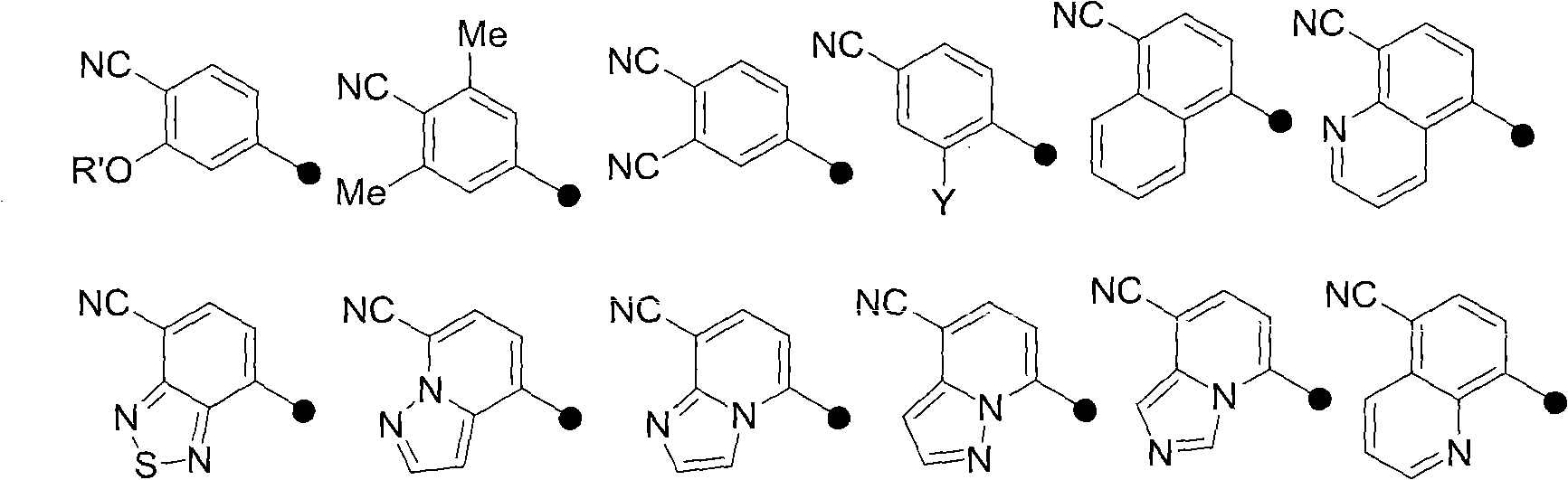
Androgen receptor antagonist for resisting prostate cancer
A halogen and compound technology, used in antitumor drugs, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of limited efficacy, limited side effects, and nervous system toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0052] Synthesis of Example 1
[0053]
[0054] Synthesis of 4-isothiocyanate-3-trifluoromethoxy-benzonitrile (intermediate 3a)
[0055] To an aqueous suspension (30 mL) of thiophosgene (4.21 g, 36.6 mmol) was slowly added 4-amino-2-methyl-benzonitrile la (5 g, 34.9 mmol). The resulting mixture was stirred at room temperature (25°C) for one hour and extracted three times with ethyl acetate (3 x 25 mL). The organic layers were combined, washed with water (1×50 mL), dried (MgSO 4 ), and vacuum-dried to give 3a (Intermediate 7, 6.5 g, 100% yield) as a beige solid, which was directly used in the next reaction.
[0056] Synthesis of 2-fluoro-N-methyl-4-nitro-benzamide (intermediate 8)
[0057]
[0058] Thionyl chloride (15 g, 130 mmol) was slowly dropped into a solution of 2-fluoro-4-nitro-benzoic acid 7 (20 g, 110 mmol) in dimethylformamide (DMF, 20 mL) at -5°C. After stirring for 1 hour, methylamine (400 mL, 800 mmol) was added dropwise. Warm slowly to room temperat...
example 2
[0072] Example 2 was obtained by reacting intermediate 10 and 7b by a method similar to Synthesis Example 1 (yield: 23%), and 7b was obtained from the corresponding aniline by a method similar to Synthesis 7a. 1 H NMR (CDCl 3 , 500MHz): δ (ppm) 8.30 (1H, dd, J = 8.6, 3.5Hz), 7.57 (3H, m), 7.26 (1H, m), 7.15 (1H, dd, J = 12.0, 2.0Hz), 6.71(1H, br s), 3.00(3H, d, J=5Hz), 2.65(2H, m), 2.52(2H, m), 2.27(1H, m), 1.70(1H, br m). Mass spectrum: 427(M+H + ).
example 3
[0076] Example 3 was obtained by the reaction of intermediate 10 and 7c (yield: 21%) by a method similar to Synthesis Example 1, and 7c was obtained from the corresponding aniline by a method similar to Synthesis 7a. 1 H NMR (CDCl 3 , 500MHz): δ (ppm) 8.30 (1H, dd, J = 8.6, 3.5Hz), 7.67 (2H, m), 7.38 (1H, d, J = 8.0Hz), 7.26 (1H, m), 7.20 ( 1H, dd, J = 11.5, 2.0Hz), 6.71 (1H, br s), 3.00 (3H, d, J = 5Hz), 2.65 (2H, m), 2.52 (2H, m), , 2.15 (3H, s), 2.15 (1H, m), 1.68 (1H, br m). Mass spectrum: 423 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com