Modified amino acid derivatives for the treatment of neurological diseases and selected psychiatric disorders
A neurodegenerative and disease technology, used in neurological diseases, drug combinations, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of compounds according to the invention.
[0061] Analytical method:
[0062] Using a JEOL-500 spectrometer (JEOL USA, Inc.MA, USA), the proton magnetic resonance ( 1 H NMR) and carbon NMR ( 13 C NMR) spectrum. With respect to TMSδ=0 ( 1 H) Delta values (ppm) report chemical shifts. J values are expressed in Hertz (Hz). deuterated chloroform (CDCl 3 ) is used as a solvent. The following signal abbreviations have been used in spectral descriptions: s (singlet), br s (broad singlet), d (doublet), t (triplet), q (quartet), m (multiplet). The UPLC / MS analysis system consists of the following: Waters The apparatus (Waters Corporation, Milford, MA, USA) was coupled to a Waters TQD mass spectrometer operating in electrospray ionization (ESI) mode. Chromatographic separation was performed using an Acquity UPLC BEH C18 (2.1 x 100 mm, 1.7 μm) column. The column was kept at 40 °C and eluted with a gradient of eluent A from 95% to 0% in 10 min a...
Embodiment 2
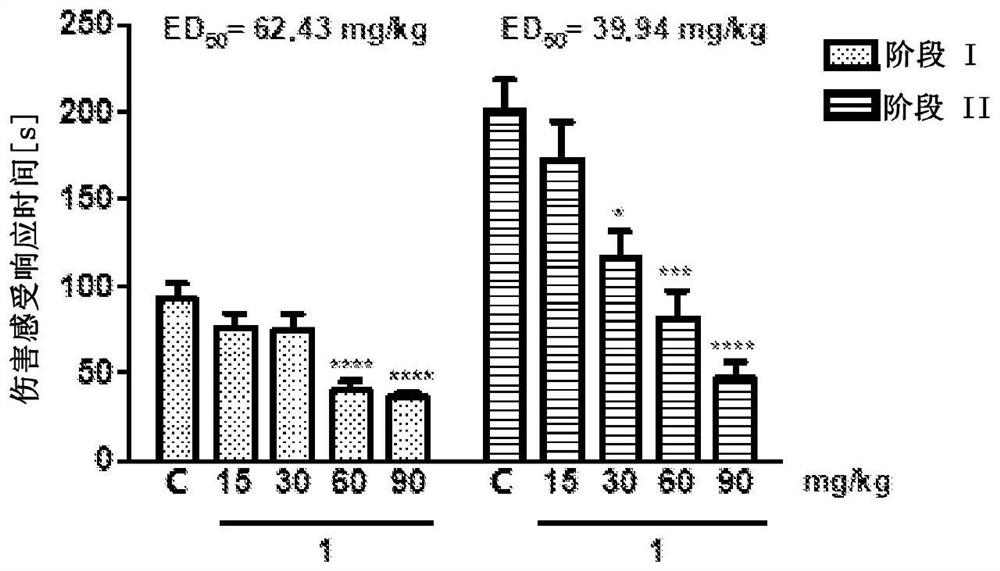
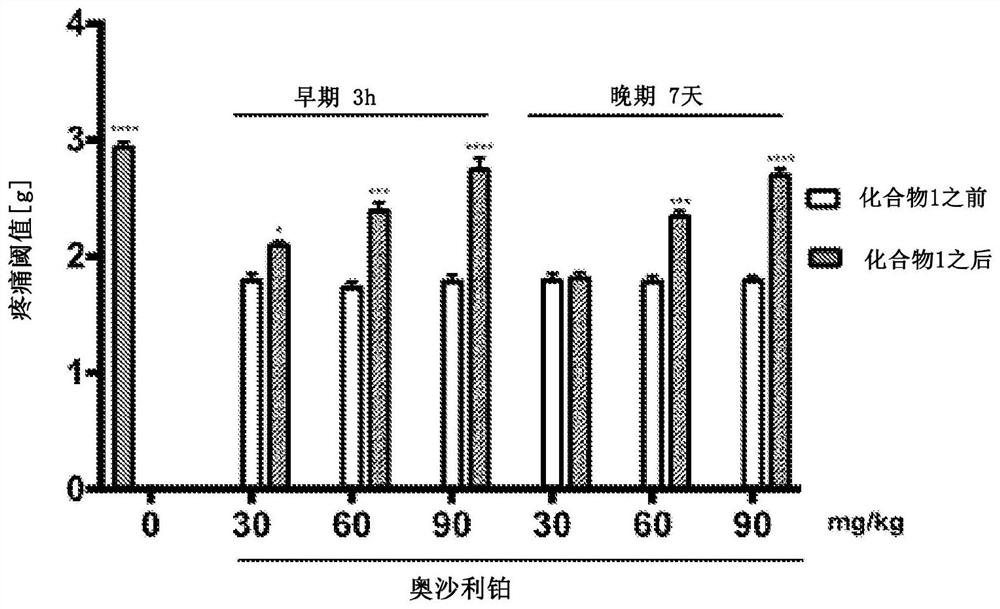
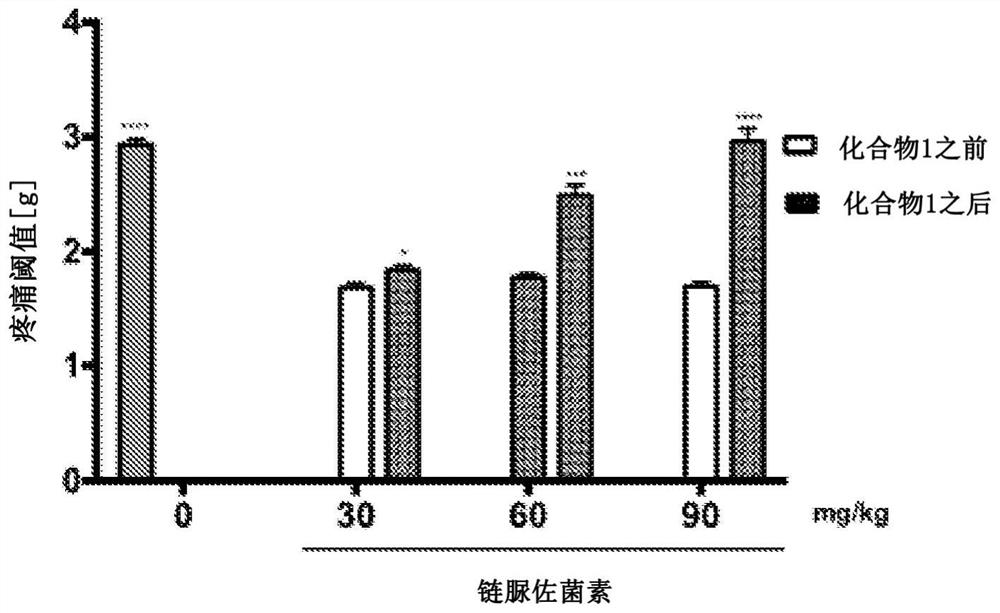
[0095] Example 2 Biological activity of compounds according to the invention.
[0096] in vivo studies
[0097] This study was performed on male Swiss albino mice (CD-1) weighing 18-26 g. After obtaining appropriate institutional approval, implement all procedures in accordance with applicable Polish and international ethical guidelines for animal testing. Substances 1-2 were administered intraperitoneally (i.p.) 30 minutes before a given test, after suspension in 1% aqueous Tween 80 solution, as a single injection in a volume of 10 ml / kg body weight. Compound 1 was also evaluated for its anticonvulsant activity after intragastric (p.o.) administration to mice and its effect on motor coordination in the rotarod test. In these studies, after compound 1 was dissolved in a mixture of DMSO, PEG 400, water for injection (10 / 40 / 50, v / v / v), 10 ml / Compound 1 was administered in a volume of kg. The anticonvulsant activity of compound 1 was also tested as part of the US screening...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com