Formulation of l-ornithine phenylacetate
A technology of phenylacetate and ornithine, applied in the field of L-ornithine phenylacetate preparations, can solve the problems of acute-on-chronic liver failure, sudden deterioration of symptoms and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
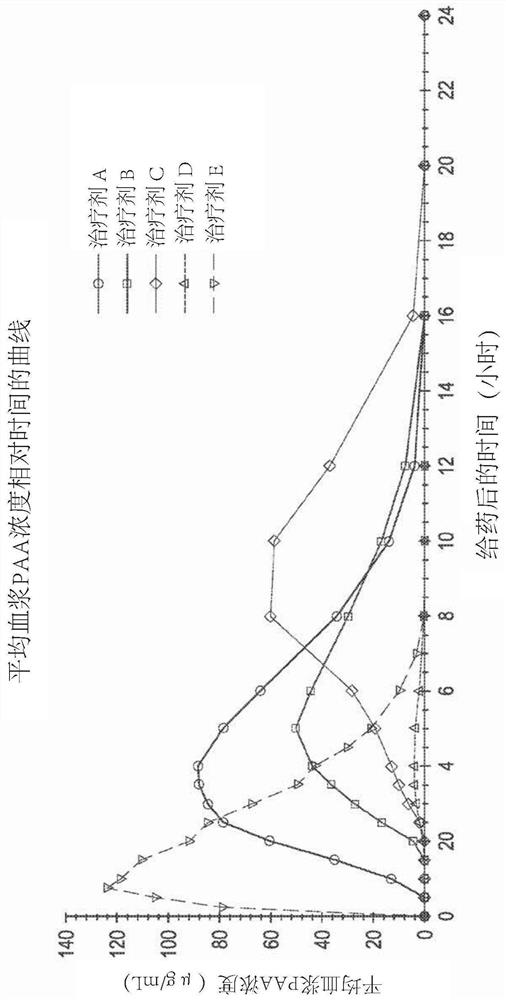
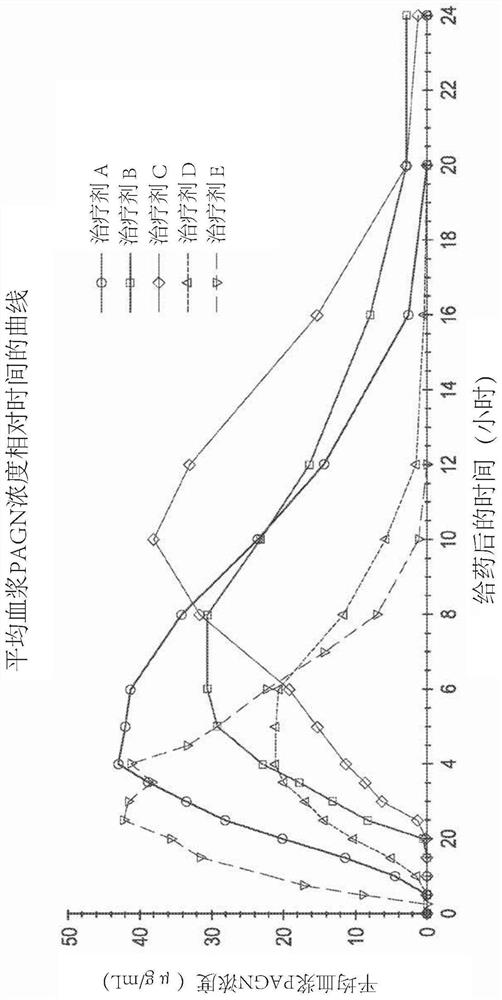
[0140] Example 1 - Phase I Pharmacokinetic Study in Healthy Humans
[0141] An open-label, five-agent, five-cycle single-dose crossover Phase I human clinical study to evaluate Pharmacokinetics of (phenylbutyrin)-phenylacetic acid prodrugs compared to phenylacetic acid and phenylacetylglutamine following a single dose of three extended-release oral dosage forms of L-ornithine phenylacetate dynamics. The study also compared the pharmacokinetics of three extended-release oral dosage forms of a single dose of L-ornithine phenylacetate compared to a single dose of L-ornithine phenylacetate in an immediate-release oral solution and security.
[0142] The five therapeutic agents are listed below: Therapeutic Agents A, B, and C each refer to a single oral dose of 10 g of Formulations A, B, and C (each equivalent to about 5 g of PAA), the components of these formulations are summarized in Table 1 below; Treatment D refers to a single oral dose of 6 mL (equivalent to about 5 g ...
Embodiment 2
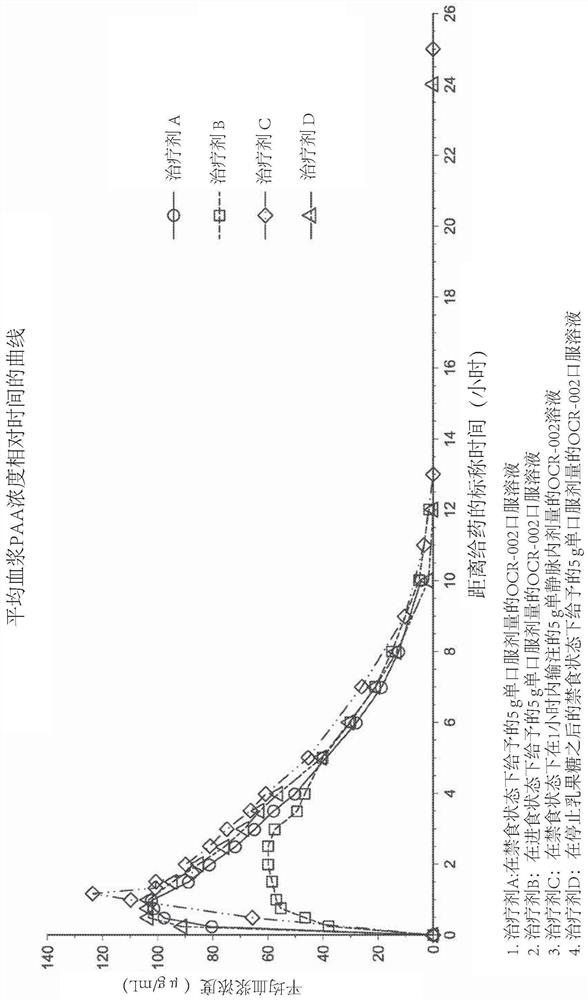
[0160] Example 2 - Phase I Pharmacokinetic Study in Child-Pugh Class A Subjects
[0161] In this example, a single-dose, partially randomized clinical study was conducted to evaluate 5 subjects with liver cirrhosis (Child-Pugh class A) in the fed state, in the fasted state, or after cessation of lactulose 5g L-ornithine phenylacetate oral solution given under fasting state. The objective was to determine whether subjects with liver cirrhosis (Child-Pugh class A) performed better in the fed, fasted state compared to a single intravenous dose of 5 g of L-ornithine phenylacetate in the fasted state. Pharmacokinetics of PAA and PAGN after administration of a single dose of 5 g of L-ornithine phenylacetate oral solution under state, or under fasting state after cessation of lactulose.
[0162] The therapeutic agents are summarized as follows: Therapeutic agent A is a 5 g single oral dose of L-ornithine phenylacetate oral solution administered in the fasted state; Therapeutic age...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com