Pyridinedicarboxamido bridged bis-β-cyclodextrin stationary phase and its preparation method and application
A pyridine dicarboxamido bridge and dicarboxamido bridge technology, applied in the field of chiral separation materials, can solve problems such as difficult separation of large-volume enantiomers, and achieve stable chromatographic performance, good reproducibility, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
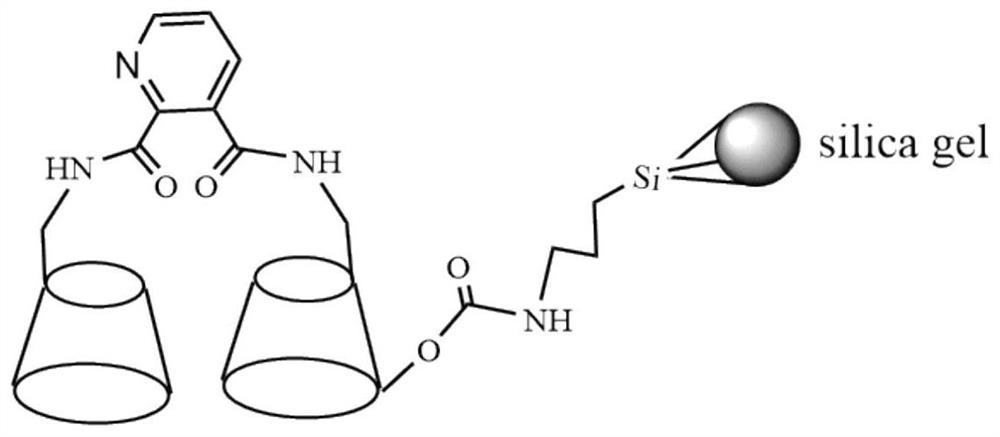
[0029] A method for preparing a pyridine-2,3-dicarboxamido bridged bis-β-cyclodextrin stationary phase, comprising the following steps:
[0030] (1) Add mono-6-amino-β-cyclodextrin, pyridine-2,3-dicarboxylic acid, DCC, HOBT, anhydrous DMF in proportions of 6.5g, 0.25g, 0.86g, 0.54g, 30mL, Stir to dissolve, magnetic stirring reaction at room temperature for 36h. The unreacted solid was filtered, the filtrate was poured into 500 mL of acetone (calculated by the amount of 0.86 g of DCC, the same below), the precipitate was precipitated and filtered to obtain a solid crude product. The solid was dissolved in a small amount of water, purified by Sephadex (C-25) flash column, the filtrate was collected and concentrated, then acetone was added to separate out the precipitate, suction filtered, and the solid was dried under vacuum at 55 °C to obtain pyridine-2,3- Dicarboxamido-bridged bis-β-cyclodextrin (PyCD) for use as a chiral ligand;
[0031] (2) PyCD, anhydrous DMF, and 3-isocy...
Embodiment 2
[0036] A method for preparing a pyridine-2,3-dicarboxamido bridged bis-β-cyclodextrin stationary phase, comprising the following steps:
[0037] (1) Add mono-6-amino-β-cyclodextrin, pyridine-2,3-dicarboxylic acid, DCC, HOBT, anhydrous DMF in proportions of 7.0g, 0.25g, 0.86g, 0.54g, and 30mL, Stir to dissolve, magnetic stirring reaction at room temperature for 36h. The unreacted solid was filtered, the filtrate was poured into 500 mL of acetone (calculated by the amount of 0.86 g of DCC, the same below), the precipitate was precipitated and filtered to obtain a solid crude product. The solid was dissolved in a small amount of water, purified by Sephadex (C-25) flash column, the filtrate was collected and concentrated, then acetone was added to precipitate the precipitate, suction filtered, and the solid was dried under vacuum at 55 °C to obtain PyCD, which was used as a chiral compound. body;
[0038] (2) PyCD, anhydrous DMF, and 3-isocyanatopropyltriethoxysilane were added ...
Embodiment 3
[0044] A method for preparing a pyridine-2,3-dicarboxamido bridged bis-β-cyclodextrin stationary phase, comprising the following steps:
[0045] (1) Add mono-6-amino-β-cyclodextrin, pyridine-2,3-dicarboxylic acid, DCC, HOBT, anhydrous DMF in proportions of 7.5g, 0.25g, 0.86g, 0.54g, 30mL, Stir to dissolve, magnetic stirring reaction at room temperature for 36h. The unreacted solid was filtered, the filtrate was poured into 500 mL of acetone (calculated by the amount of 0.86 g of DCC, the same below), the precipitate was precipitated and filtered to obtain a solid crude product. The solid was dissolved in a small amount of water, purified by Sephadex (C-25) flash column, the filtrate was collected and concentrated, then acetone was added to precipitate the precipitate, suction filtered, and the solid was dried under vacuum at 55 °C to obtain PyCD, which was used as a chiral compound. body;
[0046] (2) PyCD, anhydrous DMF, and 3-isocyanatopropyltriethoxysilane were added in p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com