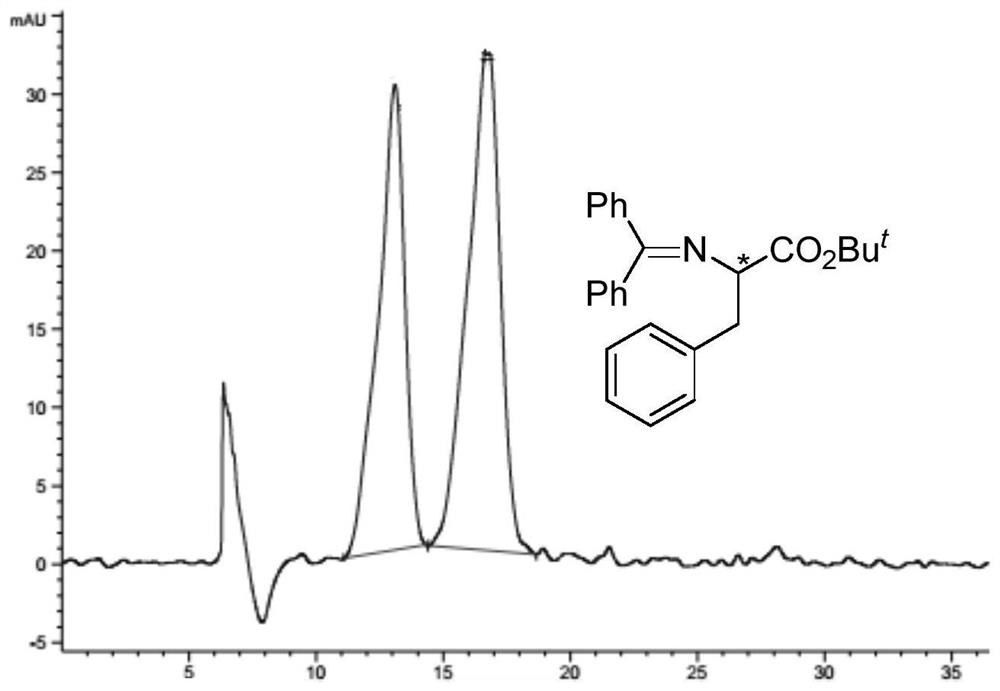
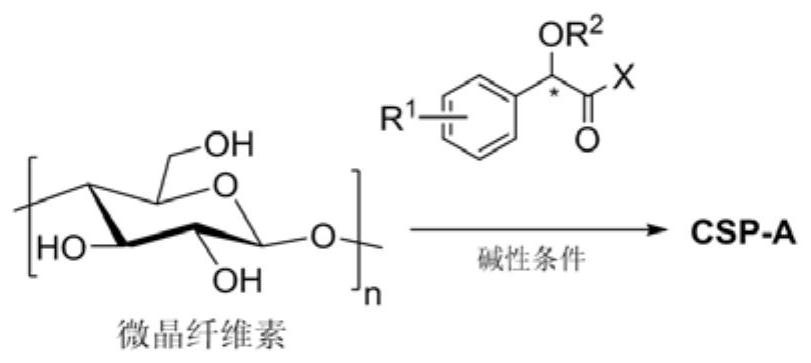
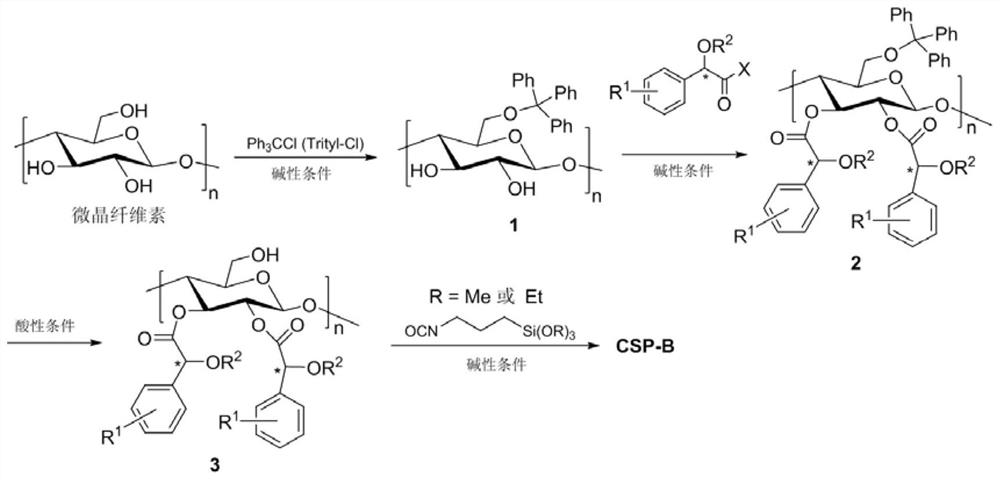
Optically pure mandelic acid derivative-cellulose chiral stationary phase, preparation method and application
A technology of cellulose and mandelic acid, which is applied in the field of chiral chromatographic separation, can solve the problems that have not been reported in the literature, and achieve the effects of low cost, good selectivity, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1.2,3, the synthesis of 6-trimandelic acid acyl cellulose CSP-A1 ( Figure 4 )
[0091] Steps:
[0092] Under argon atmosphere, (S)-mandelic acid (compound 1, 15.20 g, optical purity >98%) was suspended in 15 mL of dry pyridine and 200 mL of dry dichloromethane (CH 2 Cl 2 ), stirred and cooled to -5°C. After 15 minutes, slowly drop in 7.5mL of freshly distilled acetyl chloride (AcCl) with a syringe, and the dropwise addition is completed within 10 minutes. The reaction solution naturally rose to room temperature, stirred for 8 h, evaporated the solvent under reduced pressure, added 200 mL of dichloromethane to the obtained residue, and washed with deionized water (50 mL), saturated aqueous ammonium chloride solution (50 mL), and deionized water (50 mL) successively. , saturated brine (50mL), washed with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 19.2 grams (99% yield) of a light yellow viscous product, which ...
Embodiment 2
[0094] Embodiment 2.2,3, the synthesis of 6-trimandelic acid acyl cellulose CSP-A2 ( Figure 5 )
[0095] Steps:
[0096] It is only necessary to replace the acetyl chloride in Example 1 with benzoyl chloride, and the rest of the operation steps are the same as in Example 1 to obtain 2,3,6-trimandelic acid acyl cellulose CSP- A2 (9.2g), placed in a vacuum oven for later use. Infrared analysis IR(cm -1 ): 1720 (C=O), 1650 (Ar), 1555 (Ar).
Embodiment 3
[0097] Embodiment 3.2,3, the synthesis of 6-trimandelic acid acyl cellulose CSP-A3 ( Image 6 )
[0098] Steps:
[0099] It is only necessary to replace the acetyl chloride in Example 1 with 4-methylbenzoyl chloride, and the rest of the operation steps are the same as in Example 1, and the 2,3,6-trimandelic acid acyl group used for coating chiral column packing can be obtained Cellulose CSP-A3 (9.7g), placed in a vacuum oven for later use. Infrared analysis IR(cm -1 ): 1726 (C=O), 1676 (Ar), 1535 (Ar).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com