Polymerization catalyst system and method for preparing highly reactive polyisobutylene
A technology for polymerization catalyst and polyisobutene, applied in the field of preparing high-reactivity polyisobutene, can solve problems such as expensive process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
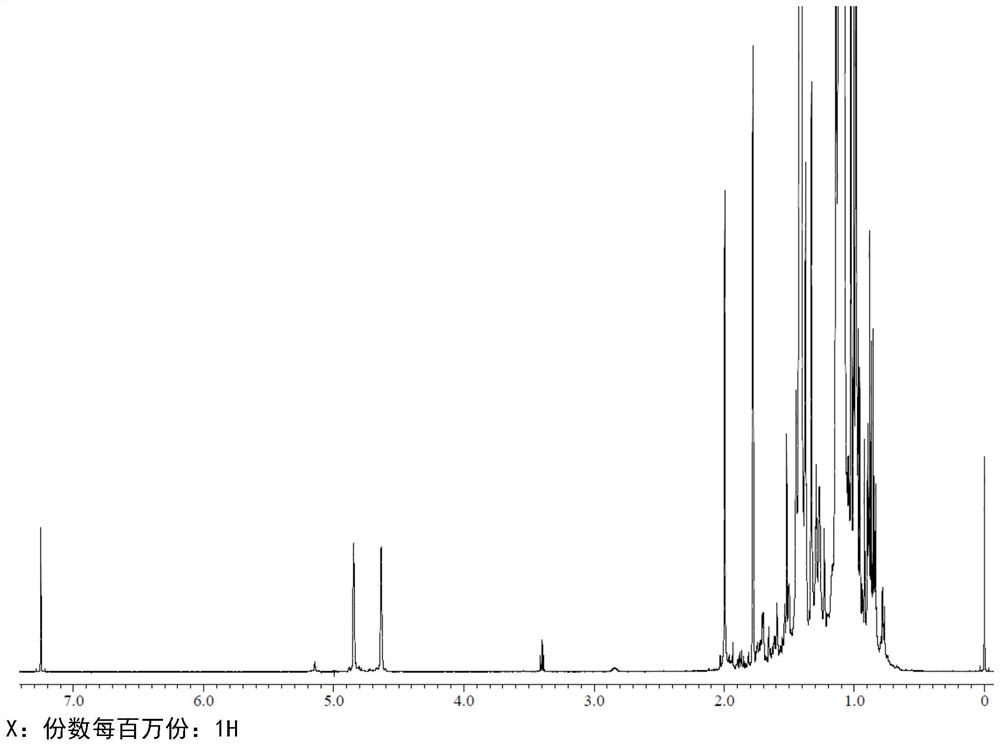
[0094] Polymerization was carried out at -10°C under a nitrogen atmosphere in a jacketed reactor equipped with a condenser. In a typical example of the experiment, 800 mL of hexane was placed in the reactor at 0°C. 200 mL of IB (4M) was condensed and added to the polymerization reactor containing hexane. Then, by adding 0.01M AlCl 3 Ether complexes (where [AlCl 3 ]=0.01M, [i-Pr 2 O]=0.005M and [Bu 2 O] = 0.005M) was added to the reactor at 0°C and polymerization started under stirring conditions. After a polymerization time of 1 hour, the polymerization was terminated by adding methanol. A polymerization yield of 98% was observed. The product has a Mn, NMR = 1500 Daltons and an exo-olefin content of 78%.
[0095] Polymerization yields were determined gravimetrically. The distribution and molecular weight (Mn, NMR) of the different olefin end groups were determined by 1HNMR.
Embodiment 2
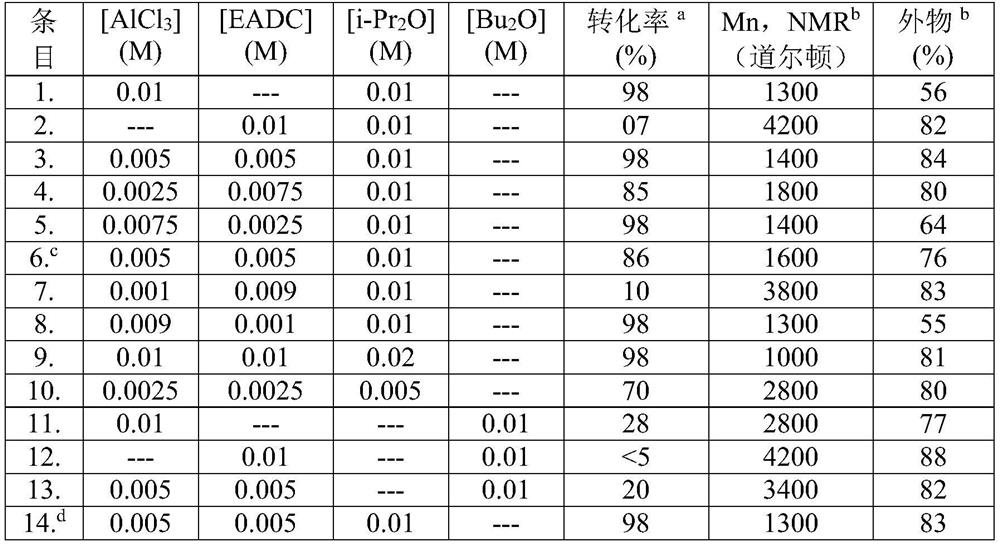
[0097] Polymerization was carried out at -10°C under a nitrogen atmosphere in a jacketed reactor equipped with a condenser. In a typical example of the experiment, 800 mL of hexane was placed in the reactor at 0°C. 200 mL of IB (4M) was condensed and added to the polymerization reactor containing hexane. Then, by adding 0.01M prepared Lewis acid·i-Pr 2 O complexes (where [AlCl 3 ]=0.005M, [EADC]=0.005M and [i-Pr 2 O] = 0.01M) was added to the reactor at 0°C and polymerization started under stirring conditions. After a polymerization time of 1 hour, the polymerization was terminated by adding NaOH solution. A polymerization yield of 98% was observed. The product has a Mn, NMR = 1500 Daltons and an exo-olefin content of 80%.
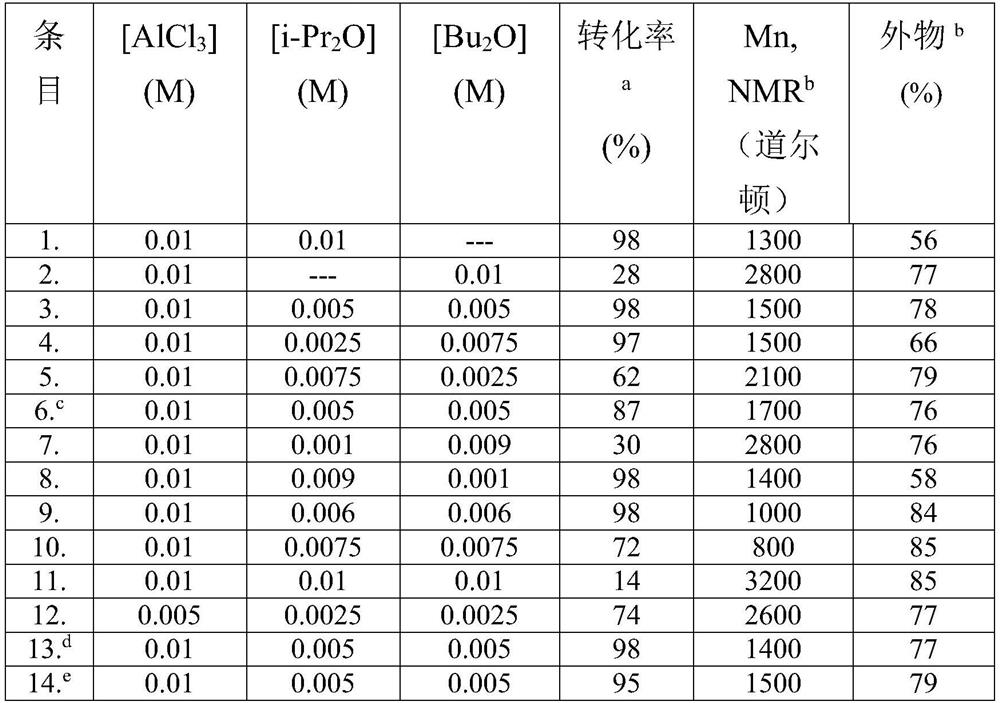
[0098] Table 1: Polymerization of IB using [Lewis acid · Lewis base] = 0.005 to 0.01 M in dry hexane at 0°C, where [Lewis acid] / [Lewis base] = 0.5 to 1.0
[0099]
[0100] a Determined by gravimetric analysis based on [IB] (Macromolecules, 201...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com