Fluorescent high-phase-change coordination compound as well as preparation method and application thereof
A coordination compound and phase change technology, which is applied in the field of fluorescent materials and phase change materials, can solve the problems of high cost, complicated process, and no fluorescence effect, and achieve the effects of easy operation, good chemical stability, and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
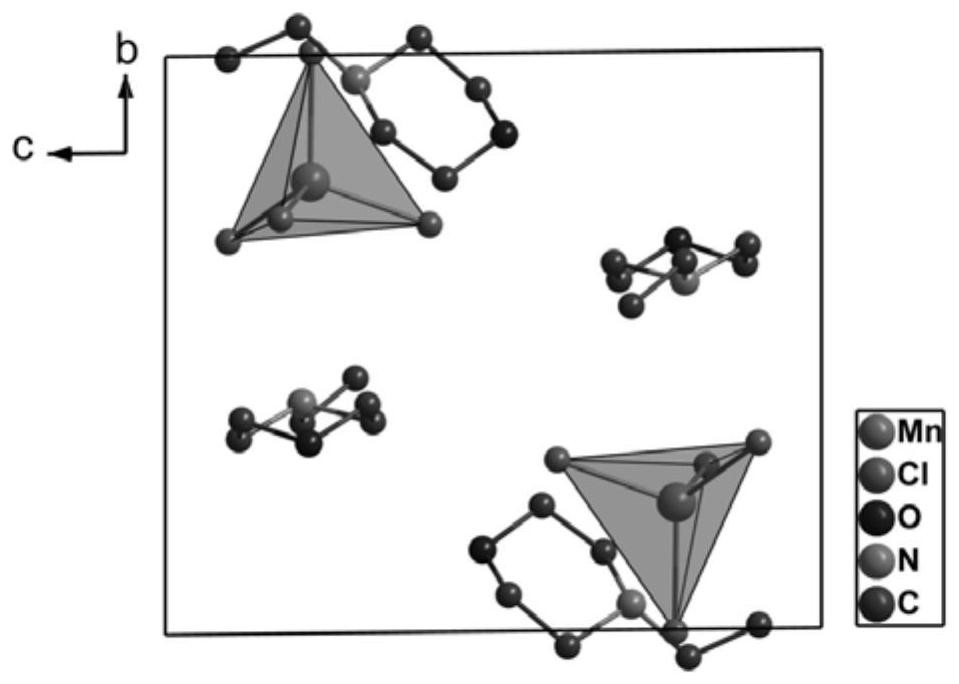
[0039] At room temperature, accurately weigh 5 mmol of manganese chloride and dissolve in 10 mL of deionized water to obtain a manganese chloride solution. Then weigh 10 mmol of N-ethylmorpholine and dissolve it in 10 mL of deionized water, and add 1.5 mL of hydrochloric acid to acidify it to obtain N-ethylmorpholine solution. Mix the above-mentioned manganese chloride solution and N-ethylmorpholine solution, stir at room temperature for 3 hours, place it at room temperature to volatilize naturally, and after 5 days, a green blocky coordination compound (C 6 h 14 NO) 2 MnCl 4 .
Embodiment 2
[0041] As in Example 1, at room temperature, accurately weigh 10 mmol of manganese chloride and dissolve it in 10 mL of deionized water to obtain a manganese chloride solution. Then weigh 10 mmol of N-ethylmorpholine and dissolve it in 10 mL of deionized water, and add 2 mL of hydrochloric acid to acidify it to obtain an aqueous solution of N-ethylmorpholine. Mix the above-mentioned manganese chloride aqueous solution and N-ethylmorpholine aqueous solution, after stirring at room temperature for 4 hours, place it at room temperature to volatilize naturally, and after 5 days, a green blocky coordination compound (C 6 h 14 NO) 2 MnCl 4 .
Embodiment 3
[0043] As in Example 1, at room temperature, accurately weigh 20 mmol of manganese chloride and dissolve it in 20 mL of deionized water to obtain an aqueous solution of manganese chloride. Then weigh 15 mmol of N-ethylmorpholine and dissolve it in 10 mL of deionized water, and add 1.8 mL of hydrochloric acid to acidify it to obtain an aqueous solution of N-ethylmorpholine. Mix the above-mentioned manganese chloride aqueous solution and N-ethylmorpholine aqueous solution, stir at room temperature for 3.5 hours, place it at room temperature to volatilize naturally, and after 5 days, a green blocky coordination compound (C 6 h 14 NO) 2 MnCl 4 .
[0044] By comparing the above-mentioned Examples 1-3, under the same conditions of temperature, ligand and metal salt, only the molar mass ratio of the solute is the same, and the solvent volume ratio is 1:1, that is, under the conditions of Example 2, the obtained The obtained crystal coordination compound (C 6 h 14 NO) 2 MnCl 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com