Abiraterone prodrugs
A technology of abiraterone and pharmacy, applied in the field of parenteral preparations, and can solve the problem of no abiraterone and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
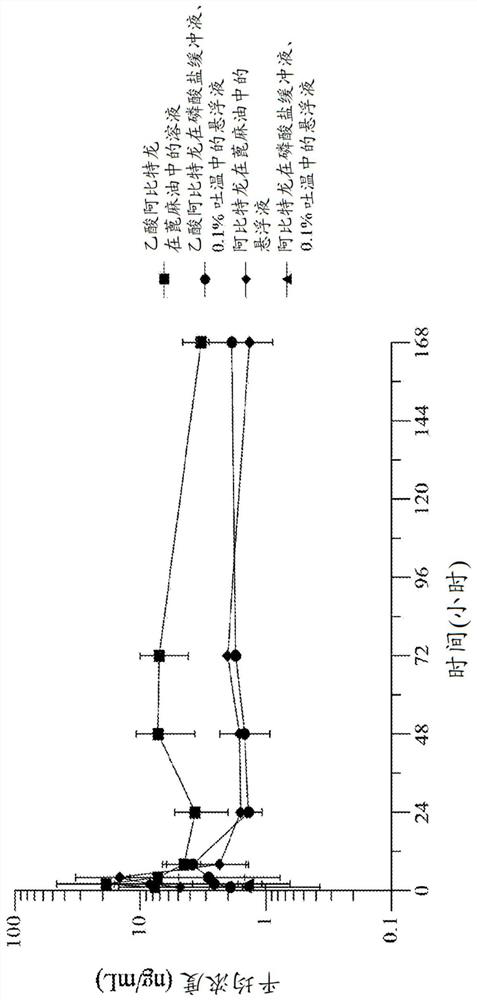
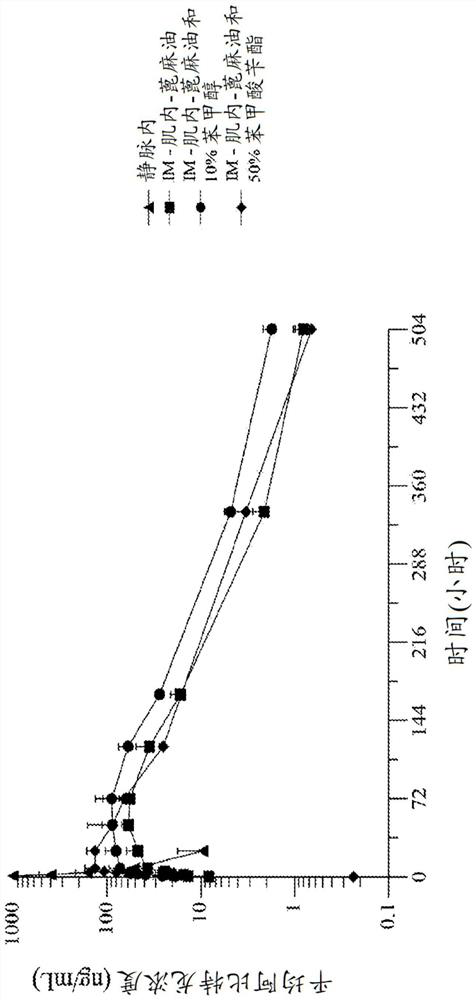
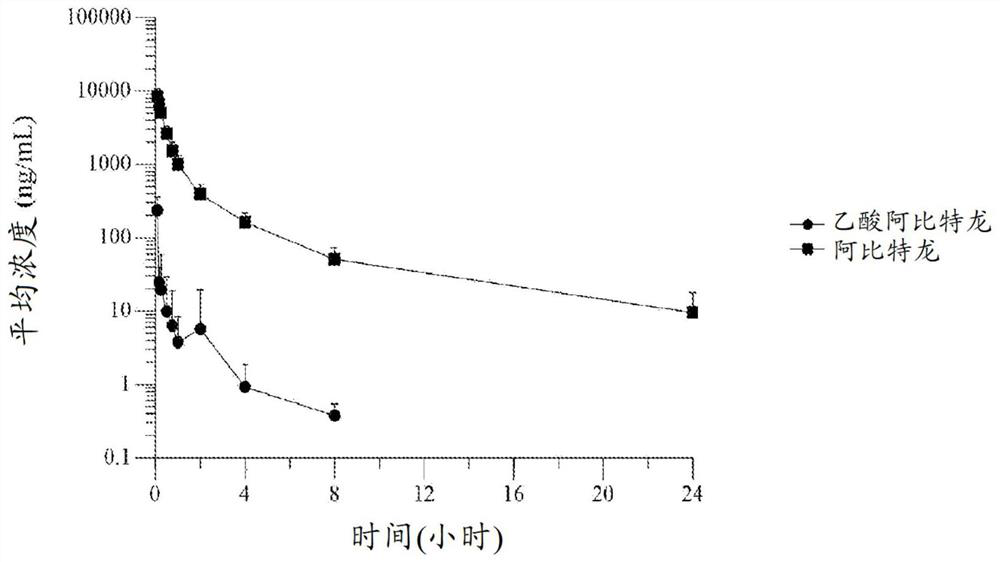
[0238] Embodiment 1. A formulation of abiraterone suitable for parenteral administration to a subject with a hormone-dependent benign or malignant disease comprising:
[0239] (a) one or more lipophilic ester forms of abiraterone, and
[0240] (b) one or more pharmaceutically acceptable carriers, diluents or excipients,
[0241] Wherein after said administering to said subject, said abiraterone prodrug formulation reaches a therapeutic plasma concentration of abiraterone.
Embodiment approach 2
[0242] Embodiment 2. The abiraterone prodrug formulation of embodiment 1, wherein said abiraterone has a therapeutic plasma concentration of at least 1.0 ng / ml.
Embodiment approach 3
[0243] Embodiment 3. The abiraterone prodrug formulation of embodiment 1, wherein said abiraterone has a therapeutic plasma concentration of at least 8 ng / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com